Ebstein's anomaly is characterised by apical displacement of the implantation of the tricuspid valve’s septal and posterior leaflets by over 0.8 cm/m2 inside the RV. While both these leaflets exhibit reduced mobility due to shortening and progressive fibrosis of the chordae, the anterior leaflet generally remains flexible, mobile and normally positioned, although elongated and fenestrated. The abnormal orifice of this tricuspid valve directs the diastolic flow toward the RVOT and not toward the apex of the RV. The right ventricular chamber is small and the RV inlet becomes part of the right atrium. It is consequently described as being “atrialised”. The RA is highly dilated. An ASD is present in > 50% of cases. The RV is highly restrictive despite its rounded appearance. It is functionally insufficient (Video) [2].
The tricuspid valve may be insufficient, stenotic or both (Figure 14.41).
Accessory conduction bundles are common, hence the high incidence of Wolff-Parkinson-White syndrome and perioperative atrial arrhythmias (25% of cases). The disease manifests clinically as a limitation of the right ventricular flow: systemic stasis, hepatomegaly and right-sided congestive failure. Cyanosis occurs if the RAP is elevated, prompting an R-to-L shunt through the ASD or PFO. Prognosis is linked to functional status, the extent of right-sided failure, the severity of arrhythmia and any cyanosis sign [4].
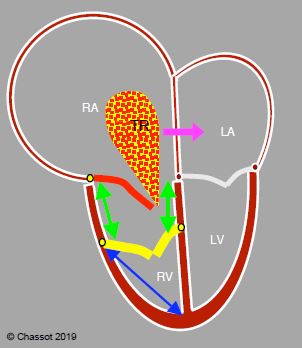
Figure 14.41: Ebstein anomaly. The septal and posterior leaflets of the tricuspid valve (in yellow) are inserted low (> 1.5 cm below the insertion of the mitral valve), while the anterior leaflet (in red) is in its normal position. The RV inlet is in the RA (green arrows). The RA is highly dilated and the RV highly restrictive. Tricuspid regurgitation (TR) is generally severe. If a PFO or ASD is present, the shunt is often R-to-L and cyanotic (pink arrow) because the RA is under high pressure. The ratio between RV atrialisation length (green arrows) and RV functional chamber length (blue arrow) must be < 0.5 in order to perform valve repair, whereby the anterior leaflet is used as the sole means of closing the valve.
The size of the trabeculated portion of the RV determines the severity of the disease and the type of possible surgical correction. If the RV is satisfactory, the operation involves reduction atrioplasty and tricuspid valve repair transforming the tricuspid into a single-leaflet valve occluded only by the anterior leaflet, or into a bicuspid valve if only one leaflet is inserted in a low position. This is only possible if the anterior leaflet is sufficiently large and mobile and if the functional chamber of the RV is at least a third of its total chamber size. This type of repair gives the valve tissue a conical shape (cone procedure) pointing towards the apex of the RV. It is often supplemented by a repair of the tricuspid annulus using a prosthetic annulus [3]. If the RV is hypoplastic, it cannot ensure pulmonary blood flow after tricuspid repair. In such cases, adding a cavopulmonary anastomosis using the Glenn procedure (SVC-RPA) may provide satisfactory pulmonary flow while bypassing the ventricle (one and a half repair) [1]. However, in severe cases a Fontan procedure is required to completely bypass the RV.
Anaesthesia
Anaesthesia is managed based on two fundamental factors: limitation of RV volume (right-sided restrictive failure) and arrhythmias. RV ejection is facilitated by pulmonary vasodilation (see Table 14.7), slight tachycardia, and low intrathoracic pressure (low-pressure mechanical ventilation). Lowering RV afterload (pulmonary vasodilation) reduces tricuspid insufficiency. The insertion of a central line may trigger very dangerous arrhythmias since part of the right atrial wall is ventricular wall. The induction technique is selected based on the patient's haemodynamic state: ketamine, midazolam and etomidate are appropriate. Anaesthesia can be efficiently maintained by a halogenated agent (isoflurane, sevoflurane) or a midazolam infusion in case of complex reconstruction.
During weaning from CPB, inotropic support of the RV is required: dobutamine, milrinone, epinephrin, NO. Atrial and ventricular arrhythmias generally respond to amiodarone. Following surgical correction, the tricuspid flow must not be restrictive – the mean gradient through the valve must not exceed 5 mmHg – residual moderate leakage is acceptable. Complete AV blocks are not uncommon after tricuspid repair. It is not infrequent for patients to require temporary support with ECMO. A decision to take such action must be made early, prior to dilation and refractory RV dysfunction.
| Ebstein anomaly |
|
Implantation apicale des feuillets postérieur et septal de la tricuspide avec rétrécissement de la cavité du VD, atrialisation de sa chambre d’admission, dilatation de l’OD et insuffisance tricuspidienne
Hémodynamique : insuffisance ventriculaire droite (VD restrictif), arythmies réfractaires
Anesthésie :
- Abaisser la postcharge du VD (vasodilatation pulmonaire, IPPV à basse pression)
- Eviter la bradycardie
- Eviter l’insuffisance du VG
|
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update May 2018
References
- CORNO AF, CHASSOT PG, PAYOT M, et al. Ebstein's anomaly: one and a half ventricular repair. Swiss Med Wkly 2002; 132:485-8
- DAVLOUROS PA, NIWA K, WEBB G, GATZOULIS MA. The right ventricle in congenital heart disease. Heart 2006; 92(Suppl 1): i27-i38
- DEARANI JA, SAID SM, O'LEARY PW, et al. Anatomic repair of Ebstein's malformation: lessons learned with cone reconstruction. Ann Thorac Surg 2013; 95:220-6
- SILVERSIDES CK, KIESS M, BEAUCHESNE L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can J Cardiol 2010; 26:e80-e97
