After 65 years of ECC practice, it is curious to observe that there is still no clear consensus or evidence-based data about the most accurate values of pressure, flow and haematocrit on pump. Most often, these elements are set according to local habits or the precautionary principle. On the other hand, the criteria used to judge haemodynamic adequacy on pump, such as neurological sequelae or postoperative renal dysfunction, vary enormously depending on the studies, the definitions given and the sensitivity with which they are analysed. Optimal perfusion can be said to be the one with the best patient survival and organ function, as well as the least impairment of coagulation and inflammatory stimulation [11].
The mean arterial pressure (MAP) and the O transport equation2 (DO2 ) summarise the different haemodynamic components that ensure tissue perfusion:
Maintaining a relatively low or relatively high mean pressure has advantages and disadvantages in both cases [11].
- High pressure (MAP 80-90 mmHg):
- Better tissue perfusion (brain, kidney, digestive tract);
- Better adaptation to the needs of hypertensive, elderly and diabetic patients;
- Providing enough flow when organ's self-regulation is disturbed;
- Improved collateral flow for tissue areas at risk of ischaemia;
- Higher pump outputs possible.
- Low pressure (MAP 50-60 mmHg):
- Less trauma to the blood components;
- Less bleeding and blood return through the bronchial arteries;
- Less suctioned and injured volume in cardiotomy aspirations;
- Less cerebral embolic load;
- Better myocardial protection (reduced coronary collateral flow).
The overriding criterion is the maintenance of adequate cerebral perfusion. Cerebral perfusion pressure (CPP) is the difference between MAP and jugular venous pressure (or JVP). This means that CPP falls not only if MAP falls, but also if JVP rises; which may happen when tilting the heart to access its posterior area, poor venous return, cannula obstruction, or Trendelenburg position.
Cerebral blood flow remains stable and independent of MAP within limits of selfregulation, outside which it becomes linearly dependent on blood pressure. Usually, the MAP considered as the lower limit is 50 mmHg because it corresponds to the physiological limit of cerebral selfregulation. However, it has been demonstrated that this limit is highly variable between individuals and pathologies, and that its mean value is 66 mmHg in bypass surgery [7,13]. Selfregulation is severely impaired in 20-25% of patients undergoing bypass surgery [13], but it is also impaired after stroke or in untreated diabetics and hypertensives [6,8]. On the other hand, cerebral selfregulation is maintained up to 25-28°C in alpha-stat mode over a pressure range of 30-100 mmHg, but is abolished in pH-stat mode or below 25°C [10,17]. As a result, neurological outcomes tend to be better in alpha-stat mode and in moderate hypothermia [15].
A MAP of >75 mmHg reduces the risk of cerebral hypoperfusion in elderly, arteriosclerotic, polyvascular or hypertensive populations, and improves collateral flow in embolic stroke [11]. A randomised clinical trial has clearly demonstrated that neurological outcomes are better when MAP is 80-100 mmHg rather than 50-60 mmHg (neurological complication rate 4.8% versus 12.9%) [4]. The same is true of renal function, which is better preserved if MAP is ≥ 80 mmHg. The optimal blood pressure is also a function of temperature. It is 60-80 mmHg above 29°C and 50 mmHg at 28°C; 40-50 mmHg is acceptable below 28°C [4].
As the organ most dependent on O2 , the brain is an excellent forerunner of body's needs. The observation of its O saturation2 (ScO2 ) by an external sensor (NIRS: near infrared spectroscopy) is an advanced signal of the state of O transport2 (DO2 ) by the circulatory system (see Chapter 6 Cerebral oximetry). But the cerebral circulation is self-regulated over a wide range of arterial pressures, making ScO2 independent of haemodynamics. Outside this area, however, the ScO2 becomes dependent on MAP. The lower limit of cerebral selfregulation is a good criterion for the lower limit of tolerable pressure; unfortunately it varies from 45 to 80 mmHg between individuals [2]. On the other hand, selfregulation is impaired in bypass surgery in 20-25% of patients during hypothermia (< 32°C) and in about 50% during rewarming [6,13]. By linking changes in MAP to changes in cerebral blood flow (transcranial Doppler) and changes in ScO2 , it is possible to determine the limits of a patient's selfregulation. Within the range of selfregulation, there is no correlation between MAP and brain flow or ScO2 ; the correlation coefficient is zero. When MAP is below the selfregulatory threshold, there is a correlation and the index tends towards 1 [2,7]. It can thus be demonstrated that the magnitude of the deviation of MAP below the autoregulatory threshold (index > 0.4) and the time spent in this zone of hypotension are correlated with the risk of stroke, renal failure and ventricular decompensation (OR 1.36-6.57) [6,12]. While it is not possible to determine the selfregulatory threshold for each individual patient, a fall in ScO2 of 20% below baseline indicates a dangerous drop in DO2 and therefore the need to increase MAP, pump output and/or haematocrit.
In conclusion, it is not possible to make a general recommendation that is valid in all circumstances, as MAP must be adapted according to the case, the temperature, the depth of anaesthesia and the surgical constraints. It is better to have a high MAP (70-80 mmHg) in a number of situations: elderly or polyvascular patients, hypertension, aortic atheromatosis, diabetes, neurological or renal diseases. In low risk situations, MAP can be maintained at about 50-60 mmHg.
Pump flow rate
There is also no magic value for pump flow, but it is usually set at 2.4 L/min/m2 (70 mL/kg/min) in normothermia (> 35°C) with an Ht of 30-40%. Although this value is in the low range of the normal cardiac index, the aim is to adapt the metabolic needs of the tissues and the perfusion pressure in the organs. The former are decreased by anaesthesia and hypothermia, allowing the flow rate to be lowered without compromising tissue balance. Thus, the recommended flow rate is 1.8 L/min/m2 at 28° and 1.0 L/min/m2 at 20° [11]. Blood flow is maintained constant in the brain by selfregulation between 1.0 and 2.5 L/min/m2 , whereas it decreases from 2.0 L/min/m2 in the liver and viscera [11]. Outside of the selfregulatory range, the flow becomes strictly pressure-related.
Using the formula for calculating resistance, one can easily assess the state of systemic arterial resistance:
SAR = (MAP - CVP) - 80 / CO
In this case, CVP is considered to be zero since RA is discharged and venous reservoir is at atmospheric pressure. Cardiac output (CO) is replaced by pump output (PO)(value given by the machine).
Therefore: SAR = MAP - 80 / PO
Pressure and flow are in balance through peripheral arterial resistance. They should not be adjusted to the detriment of each other. Thus, it does not make sense for the perfusionist to lower the pump output below the physiological limit because the pressure is too high, or for the anaesthetist to administer a vasoconstrictor to raise the pressure when the output is too low. In the rewarming phase, it is important to maintain a high flow rate and some degree of vasodilation to promote tissue heat exchange. In aortic insufficiency (AI) or left-to-right shunt, pressure should be maintained by flow alone until the aorta or shunt is clamped; administration of vasopressor will only worsen the AI or increase the shunt. The interplay of arterial resistance, pump flow and perfusion pressure therefore requires constant collaboration and communication within the patient's team.
Transport of O 2
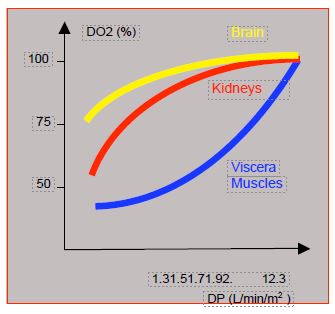
Oxygen consumption (VO2 ), which is normally 150 mL/min/m2 , falls to 120-140 mL/min/m2 under normothermic anaesthesia and to 45 mL/min/m2 at 27° [3]. In bypass surgery, curarisation can reduce it by a further 20-30% [5]. Thus, the recommended flow rate at 28°C is 1.8 L/min/m2 , and at 20°C 1.0 L/min/m2 . Evidence of flow adequacy is provided by a SvO2 ≥ 70%. O transport2 (DO2 = PO - (Hb - SaO2 - 1.36) + 0.003 PaO2 ) can be improved by increasing the pump flow (PO), FiO2 , or Ht (transfusion, haemoconcentration by ultrafiltration). The critical DO2 is the value below which O2 consumption (VO2 ) becomes dependent on cardiac output (see Figure 5.72). In sleeping humans it is 330 mL/min/m2 and in bypass surgery, if present, 280-300 mL/min/m2 [14]. DO2 and VO2 remain normal over a range of Ht from 39% to 25% [9]. An DO2 < 270 mL/min/m2 on bypass is the best predictor of postoperative renal failure [16]. However, there is a hierarchy of organs in flow dependence. Thus, the DO2 remains satisfactory in the brain and kidneys when the flow rate drops to 1.4 L/min/m2 , whereas it is already insufficient in the viscera and muscles when the flow rate drops below 1.7-2.0 L/min/m2 [1]. The viscera are therefore at high risk of ischaemia because they do not have an selfregulatory system (Figure 7.24).
Figure 7.24: Changes in O supply2 (DO2 ) to different organs as a function of pump flow (PF). The DO2 is well maintained in the brain, and relatively well in the kidneys, up to a flow of 1.4 L/min/m2 , but decreases as soon as flow is below 2.0 L/min/m2 in the viscera and muscles [1]. The latter therefore suffer from ischaemia while the brain functions normally.
The problem of haematocrit is discussed in the section on haemodilution (see Priming fluid).
| Hemodynamics and ECC |
|
MAP should be adjusted according to the case, temperature, depth of anaesthesia and surgical constraints. It should be high (70-80 mmHg) in a range of situations: elderly or polyvascular patients, hypertension, aortic atheromatosis, diabetes, neurological or renal diseases. The average value varies between 50-60 mmHg (low risk patients) and 70-80 mmHg (high risk patients).
The pump flow is usually set at 2.4 L/min/m2 (70 ml/kg) in normothermia (> 35°C) at a Ht of 30-40%. In hypothermia it can be lowered to 1.8 L/min/m2 at 28°C and 1.0 L/min/m2 at 20°C, but should be increased to compensate for Ht if it is too low. Flow is adequate when the SvO2 is ≥ 70%.
In bypass surgery, the DO2 becomes dependent on pump flow below 300 mL/min/m2 . The DO2 remains satisfactory in the brain and kidneys when flow drops to 1.4 L/min/m2 , whereas it is already insufficient below 1.7-2.0 L/min/m2 in the viscera and muscles, which do not benefit from an selfregulation system.
|
CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- BOSTON US, SLATER JM, ORSZULAK TA, et al. Hierarchy of regional oxygen delivery during cardiopulmonary bypass. Ann Thorac Surg 2001; 71:260-4
- BRADY K, JOSHI B, ZWEIFEL C, et al. Real-time continuous monitoring of cerebral blood flow selfregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010; 41:1951-6
- FIACCADORI E, VEZZANI A, COFFRINI E, et al. Cell metabolism in patients undergoing major valvular surgery: relationship with intra and postoperative hemodynamics, oxygen transport, and oxygen utilization patterns. Crit Care Med 1989; 17:1286-91
- GOLD JP, CHARLESON MD, WILLIAMS-RUSSO P, et al. Improvement in outcomes after CABG. A randomized trial comparing intraoperative high versus mean arterial pressure. J Thorac Cardiovasc Surg 1995; 110:1302-11
- IRISH CL, MURKIN JM, CLELAND A, et al. Neuromuscular blockade significantly decreases systemic oxygen consumption during hypothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1991; 5:132-7
- JOSHI B, BRADY K, LEE J, et al. Impaired selfregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg 2010; 110:321-8
- JOSHI B, ONO M, BROWN C, et al. Predicting the limits of cerebral selfregulation during cardioplumonary bypass. Anesth Analg 2012; 114:503-10
- LANIER WL. Glucose management during cardiopulmonary bypass: cardiovascular and neurologic implications. Anesth Analg 1991; 72:423-7
- LIAM BL, PLOCHL W, COOK DJ, et al. Hemodilution and whole body balance during normothermic cardiopulmonary bypass. J Cardiothorac vasc Surg 1998; 115:1203-8
- MURKIN JM, MARTZKE JS, BUCHAN AM, et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. II Neurologic and cognitive outcomes. J Cardiothorac Vasc Surg 1995; 110:349-55
- MURPHY GS, HESSEL EA, GROOM RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg 2009; 108:1394-417
- ONO M, BRADY K, EASLEY RB, et al. Duration and magnitude of blood pressure below cerebral selfregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg 2014; 147:483-9
- ONO M, JOSHI B, BRADY K, et al. Risks for impaired cerebral selfregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth 2012; 109:391-8
- PAROLARI A, ALAMANNI F, GHERLI T, et al. Cardiopulmonary bypass and oxygen consumption: oxygen delivery and hemodynamics. Ann Thorac Surg 1999; 67:1320-7
- PATEL RL, TURTLE MR, CHAMBERS DJ, et al. Alpha-stat acid-base regulation during cardiopulmonary bypass improves neuropsychologic outcomes in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 1996; 111:1267-79
- RANUCCI M, ROMITTI F, ISGRO G, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg 2005; 80:2213-20
- SUNGURTEKIN H, BOSTON US, COOK DJ. Bypass flow, mean arterial pressure, and cerebral perfusion during cardiopulmonary bypass in dogs. J Cardiothorac Vasc Anesth 2000; 14:25-8