Pulmonary atresia with ventricular septal defect is an extreme form of Fallot in which there is no communication between the RV and PA. At birth, the patent ductus arteriosus is the only source of pulmonary blood flow. A third of children maintain flow to the PA with 2 to 6 major aortopulmonary collateral arteries (MAPCA) [5]. Children experience varying degrees of cyanosis depending on the degree of R-to-L shunting via the VSD and L-to-R shunting via the patent ductus arteriosus or collaterals. Long-term survival is rarely possible under these circumstances, since the PA is hypoplastic and the collaterals do not provide sufficient flow, while the connection between the aorta and the pulmonary arteries causes structural deterioration of these vessels leading to irreversible pulmonary arterial hypertension.
If collaterals are absent, this lesion constitutes a neonatal emergency, since closure of the ductus arteriosus results in death. Total correction is performed immediately if circumstances permit: patch over the VSD and RV-PA conduit (homograft or valved bovine jugular vein conduit). If this is not possible due to the specific circumstances of the case, a stent may be inserted percutaneously in the ductus arteriosus to prevent it from closing or a Blalock-type calibrated peripheral shunt may be constructed [5]. If MAPCA are present, the surgical strategy is based on unifocalisation, which involves transforming the system of collaterals into a single mediastinal vessel vascularising the PA trunk, and calibrating this to achieve PAP of approximately 30 mmHg. Preoperative catheterisation is required for optimal mapping of these vessels to differentiate competitive MAPCA (simple ligatures) from non-competitive or distal competitive MAPCA (compulsory unifocalisation) on which vascularisation of a lobe or pulmonary segment is dependent (Figure 14.61A). Survival at 10 years is 70-85% [1].
Anaesthetists face three key issues [6]:
If collaterals are absent, this lesion constitutes a neonatal emergency, since closure of the ductus arteriosus results in death. Total correction is performed immediately if circumstances permit: patch over the VSD and RV-PA conduit (homograft or valved bovine jugular vein conduit). If this is not possible due to the specific circumstances of the case, a stent may be inserted percutaneously in the ductus arteriosus to prevent it from closing or a Blalock-type calibrated peripheral shunt may be constructed [5]. If MAPCA are present, the surgical strategy is based on unifocalisation, which involves transforming the system of collaterals into a single mediastinal vessel vascularising the PA trunk, and calibrating this to achieve PAP of approximately 30 mmHg. Preoperative catheterisation is required for optimal mapping of these vessels to differentiate competitive MAPCA (simple ligatures) from non-competitive or distal competitive MAPCA (compulsory unifocalisation) on which vascularisation of a lobe or pulmonary segment is dependent (Figure 14.61A). Survival at 10 years is 70-85% [1].
Anaesthetists face three key issues [6]:
- LV dysfunction – inotropic support (dobutamine, epinephrin-milrinone), reduction of afterload (NO);
- Intrapulmonary haemorrhage;
- Pulmonary reperfusion lesions (oedema, bronchospasm, ventilation problems); extubation is delayed and IPPV with PEEP is prolonged in the postoperative phase.
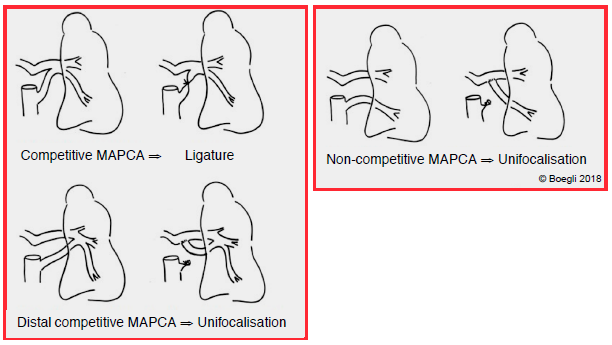
Figure 14.61A: Diagram showing various types of surgical corrections in cases of MAPCA.
Pulmonary atresia with intact septum
Pulmonary atresia with intact ventricular septum is a complete fibromuscular blockage associated with varying degrees of RV and tricuspid valve hypoplasia. In 10-20% of cases, fistulas between the RV and epicardial coronary arteries render myocardial perfusion dependent on RV function, with RV pressure significantly raised due to the blockage impeding ejection [2]. Survival is ensured by an ASD (R-to-L shunt) and a patent ductus arteriosus kept open by a prostaglandin infusion. Closure of the patent ductus arteriosus immediately exacerbates cyanosis. Several therapeutic options are available depending on the degree of RV hypoplasia [5,6].
- Well-developed RV, normal tricuspid valve, no coronary fistulas: patch to widen the RVOT in the 1st week of life, percutaneous or surgical valvulotomy if atresia is caused by a membrane.
- Underdeveloped RV, hypoplastic tricuspid valve and coronary fistulas present: systemic-pulmonary anastomosis. Firstly, Blalock shunt to ensure pulmonary blood flow. Subsequently, Glenn anastomosis (SVC - RPA) or Fontan procedure if PAP is sufficiently low – not indicated before the age of 3 months as PVR is still too high. In these specific circumstances, the RV cannot be decompressed due to the risk posed to coronary perfusion.
- Moderate forms: Blalock shunt followed by RV-PA anastomosis unless the coronary circulation is dependent on the RV.
The RV is small, hypertrophic and under high pressure. If no coronary fistulas are present, it must be decompressed to allow its growth. Anaesthesia is subject to the same constraints as in cases of single ventricle defects (see Ventricular hypoplasia).
Special case: isolated pulmonary stenosis
While pulmonary stenosis is present in approximately 10% of congenital heart disease cases, it may also exist in isolation and may manifest itself late in adolescence as right ventricular hypertrophy followed by RV failure. The RV is often ischaemic as the O2 requirement is raised while O2 supply is compromised by intraventricular hypertension during systole. An increase in afterload occurring from birth causes concentric-type hypertrophy rather than RV dilation. Right heart function remains adequate as long as intraventricular pressure is < 50% of left heart pressure and there is no associated volume overload. Patients become symptomatic if RV pressure exceeds half of systemic pressure [7]. The valve is dome-shaped with a narrowed central opening (Figure 14.61B).
Special case: isolated pulmonary stenosis
While pulmonary stenosis is present in approximately 10% of congenital heart disease cases, it may also exist in isolation and may manifest itself late in adolescence as right ventricular hypertrophy followed by RV failure. The RV is often ischaemic as the O2 requirement is raised while O2 supply is compromised by intraventricular hypertension during systole. An increase in afterload occurring from birth causes concentric-type hypertrophy rather than RV dilation. Right heart function remains adequate as long as intraventricular pressure is < 50% of left heart pressure and there is no associated volume overload. Patients become symptomatic if RV pressure exceeds half of systemic pressure [7]. The valve is dome-shaped with a narrowed central opening (Figure 14.61B).
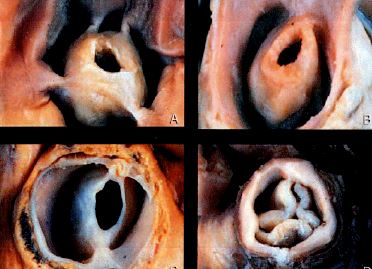
Figure 14.61B: Examples of congenital pulmonary stenosis – the valve is narrow, hypoplastic, and more or less cone-shaped [3].
Surgery is indicated if the maximum gradient is ≥ 60 mmHg and RV pressure > 0.7 times LV pressure [5]. Percutaneous valve repair by balloon dilation is the preferred treatment, but it is associated with major pulmonary insufficiency in 25% of cases. Although more invasive, surgical valvulotomy leads to excellent outcomes and 95% survival at 25 years.
Four key constraints apply to anaesthetic management of isolated pulmonary stenosis.
Four key constraints apply to anaesthetic management of isolated pulmonary stenosis.
- Risk of RV ischaemia: maintain systemic pressure with arterial vasconstrictors to ensure right coronary perfusion.
- Risk of hypovolaemia: blood flow from the hypertrophied VD is highly dependent on its preload. In addition to the stenosis effect, hypovolaemia therefore prompts a significant fall in pulmonary flow and arterial desaturation.
- Management of right systolic dysfunction: postoperatively, the hypertrophied RV may also exhibit restrictive-type diastolic dysfunction, which entails significant elevation of CVP to maintain the ventricular output [4].
- Management of arrhythmias.
Once the PA is unclamped, the sudden increase in pulmonary flow may cause significant alveolar exudate. This pulmonary reperfusion lesion occurs in a quarter of all cases. High PEEP is beneficial [8].
| Pulmonary atresia |
|
Various constraints affect perioperative management depending on the type of lesion.
- Small and hypertrophic RV with limited systolic volume and diastolic dysfunction - RV systolic dysfunction (dobutamine, milrinone, NO) - Possible dynamic component to the RVOT stenosis - Maintaining coronary perfusion pressure - Postoperative risk of reperfusion pulmonary oedema |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- AMARK KM, KARAMLOU T, O’CAROLL A, et al. Independent factors associated with mortality, reintervention, and achievement of complete repair in children with pulmonary atresia with ventricular septal defect. J Am Coll Cardiol 2006 ; 47 :1448-56
- ASHBUM DA, BLACKSTONE EH, WELLS WJ, et al. Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg 2004 ; 127 :1000-7
- BOHN D. Anomalies of the pulmonary valve and pulmonary circulation. In: LAKE CL. Pediatric Cardiac Anesthesia, 2nd edition, Appleton & Lange, Norwalk, 1993, 295-323
- CHATURVEDI RR, SHORE DF, LINCOLN C, et al. Acute right ventricular restrictive physiology after repair of tetralogy of Fallot : association with myocardial injury and oxidative stress. Ciculation 1999; 100:1540-7
- HONJO O, Van ARSDELL GS. Cardiovascular procedures : surgical considerations. In : BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1589-608
- SCHMITZ ML, ULLAH S. Anesthesia for right-sided obstructive lesions. In : ANDROPOULOS DA, et al, eds. Anesthesia for congenital heart disease. Oxford: Blackwell-Futura, 2005, 328-45
- WARNES CA. Adult congenital heart disease. Importance of the right ventricle. J Am Coll Cardiol 2009; 54:1903-10
- YACOUBY S, MEADOR M, MOSSAD E. Lung reperfusion injury in patients after balloon angioplasty for pulmonary artery stenosis. J Cardiothorac Vasc Anesth 2014; 28:502-5
