- Preload: this must be high to ensure a sufficient gradient across the mitral valve, but the pulmonary vascular bed is not very compliant and is already chronically overloaded. In the case of hypervolaemia or increased venous return (Trendelenburg position), there is a risk of florid APO. Hypovolemia is very poorly tolerated because the transmitral flow decreases in geometric proportion to the decrease in PLA and cannot be compensated by tachycardia.
- Afterload: The SAR must be kept high to compensate for the small stroke volume; the LV ejection function, which is generally preserved, can withstand this increase in afterload. Arterial vasodilatation should be avoided. An alpha vasoconstrictor is the best way to maintain systemic pressure intraoperatively.
- Contractility: LV function is preserved but there is no preload reserve; stroke volume is low and fixed. Beta-catecholamines are only used in the event of an excessive fall in CO (fall in SvO2), as they do not benefit the LV for two reasons: 1) the tachycardia reduces LV filling and 2) the increase in CO increases the trans-mitral gradient and PLA. The LV is dilated or hypertrophied depending on the degree of pulmonary hypertension; in severe PHT it is inadequate. In this case, it is the LV that benefits most from inotropic stimulation.
- Frequency: this must be kept low to allow ventricular filling, which is very slow; tachycardia implies an increase in transmitral flow to maintain systolic flow, leading to a geometric increase in PLA and pulmonary stasis. If pacing is necessary, a long P-Q interval (0.15-0.20 sec) should be considered.
- Pulmonary arterial pressure: Pulmonary hypertension is precapillary (arterial vasoconstriction) and postcapillary (venous hypertension). The risk of arterial vasoconstriction is increased in hypoxia, hypercarbia, acidosis or N2O; normobaric hyperventilation (PetCO2 30 mmHg) is desirable, avoiding high ventilation pressures to avoid increasing LV afterload. Pulmonary vasodilators are only indicated in the setting of LV failure, as increased pulmonary flow increases the risk of APO.
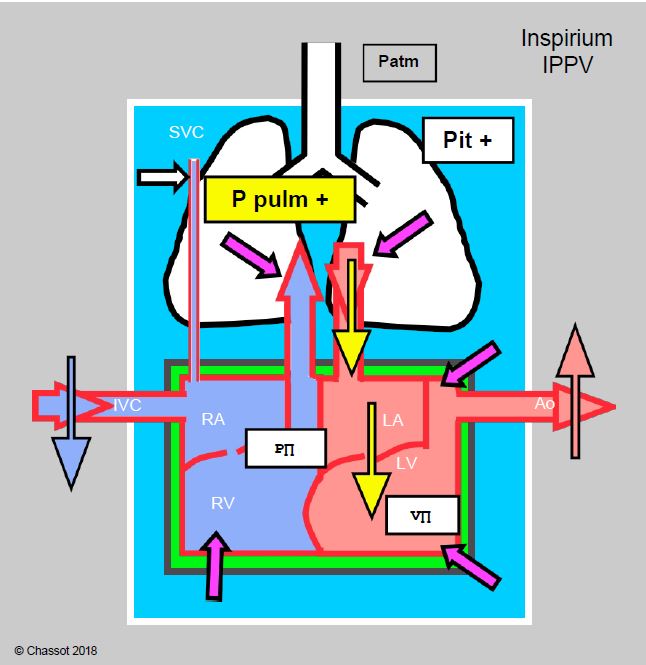
- Positive Pressure Ventilation: This is highly beneficial for left-sided flow as long as venous return to the right heart is maintained and the RV is not failing. By compressing the lungs like a sponge, positive pressure ventilation increases return to the LA, reduces transmural pressure in the atrium and improves transmitral flow by reducing stasis (Figure 11.97). The effects of IPPV are mediated by mean intrathoracic pressure.
Figure 11.97: Cardiorespiratory interactions of positive pressure ventilation (IPPV) in mitral valve disease. Schematic representation of the effects of inspiratory increases in intrathoracic pressure (Pit) with IPPVI and PEEP. The increase in Pit and intrapulmonary pressure (purple arrows) increases LV afterload, LA filling and LV preload, compresses the ventricles, slows venous return from the inferior vena cava (IVC) and facilitates LV ejection because its afterload does not change (it is outside the chest). As long as the right heart flow is compensated, the situation is better than with spontaneous ventilation because anterograde flow through the mitral valve is facilitated and pulmonary congestion is reduced.
Anaesthetic technique for mitral valve surgery in MS
- Premedication should be kept to a minimum to avoid the risk of hypoventilation and hypercapnia in case of pulmonary hypertension (PHT). If an anticholinergic is required, atropine should be avoided as it is unpredictly tachycardic.
- Induction must be slow, with as little change in baseline conditions as possible:
- Etomidate: the only agent without haemodynamic effects.
- Midazolam: reduction of central sympathetic tone leads to a slight decrease in CO.
- Propofol: reduces preload and anterograde mitral flow; only possible with reduced dose and slow administration in stable patients.
- Fentanils: beneficial (bradycardia).
- Thiopental, ketamine: excluded because of their tachycardia.
- Local anaesthesia of the larynx and trachea should be used to avoid a tachycardia-inducing episode during intubation.
- Maintain anaesthesia: avoid decrease in SAR and increase in PAR, limit saline intake:
- Sevoflurane: agent of choice.
- Perfusion of propofol or midazolam: adjust doses to ensure haemodynamic stability.
- Isoflurane: not recommended ( ↓ SAR and tachycardia).
- Desflurane: not recommended ( ↑ frequency and ↑ PAR).
- IPPV + PEEP beneficial; hyperventilation reduces PARs.
- Systemic hypotension: ↑ RAS with an alpha vasopressor (phenylephrine, noradrenaline), avoid fluid overload.
- Avoid tachycardia:
- Thiopental, ketamine and pancuronium are contraindicated.
- No ephedrine; in case of hypotension: phenylephrine, noradrenaline.
- Do not use atropine in cases of excessive bradycardia (unpredictable response); prefer ephedrine.
- Do not use phentolamine in the event of a hypertensive attack; nitroglycerine is preferable.
- After MVR tachycardia is no longer dangerous.
- Treat pulmonary hypertension: hypocapnia, alkalosis, prostacyclin, NO•. Avoid: ketamine, desflurane, N2O, stress, pain, acidosis, hypoxaemia, hypercapnia.
- Avoid hypervolaemia; Swan-Ganz pulmonary catheter is essential for fluid management (continuous monitoring of PCWP).
- Maintain contractility if necessary (deterioration in systolic function may be masked by low preload); tachycardia is no longer a concern after ECC.
- Prefer dobutamine; dopamine if dose < 5 mcg/kg/min.
- Milrinone (± epinephrine) if PHT.
- TEE monitoring:
- Degree of calcification, condition of the subvalvular apparatus (poorly visible to the operator), presence of thrombus in the LA (LAA).
- LV size and function, RV function, signs of PHT.
- Assessment of blood volume.
- Post-ECC: results of commissurotomy, prosthesis function, paravalvular leakage, LV adaptation to new preload conditions.
- Pulmonary artery catheter: useful in all cases.
- Measurement of PCWP (PHT).
- PCWP: adjustment of perfusion; reflects pulmonary capillary pressure and PLA but not LV end-diastolic pressure.
- Measurement of SV, CO and SvO2 (adequacy of CO to metabolic needs).
- ScO2 : assessment of peripheral perfusion.
Percutaneous commissurotomy
The procedure is usually performed in a cath lab or hybrid room. Patient comfort is better under general anaesthesia for two reasons [2]:
- Haemodynamic instability and risk of resuscitation;
- Presence of a transesophageal echocardiography probe.
Apart from intubation and the TEE probe, stimulation is of minor importance and lasts 1-2 hours. During the dilatation itself, the balloon inflated in the mitral lumen obstructs the flow: cardiac output is temporarily zero. Extubation is performed at the end of the procedure. The haemodynamic constraints are the same as for surgery.
| Haemodynamic sought in mitral stenosis |
| Normal and stable preload
Systemic vasoconstriction Normovolemic - Slow - Closed |
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- FROGEL J, GALUSCA D. Anaesthetic considerations for patients with advanced valvular heart disease undergoing non-cardiac surgery. Anesthesiol Clin 2010; 28:67-85
- HEMLATA, GOYAL P, TEWARI S, et al. Anaesthetic considerations for balloon mitral valvuloplasty in pregnant patient with severe mitral stenosis: a case report and review of literature. J Clin Diagn Res 2017; 11:UDO1-UDO3