It is not uncommon to find a persistent left superior vena cava (LSVC) on perioperative TEE examination. This occurs in 0.3-1% of the population and in 5% of congenital heart disease cases [2]. It generally drains into the coronary sinus, which is significantly enlarged (Figure 15.8) (Video).
Video: Dilatation of the coronary sinus in a case of left superior vena cava; the coronary sinus appears as a vertical tube near the top of the screen, draining into the right atrium; the right ventricle is hypertrophied.
The right superior vena cava (RSVC) may be present, hypoplastic or absent. Although LSVC is of no interest as a pathology, it entails risks for surgery or central venous canulation [2].
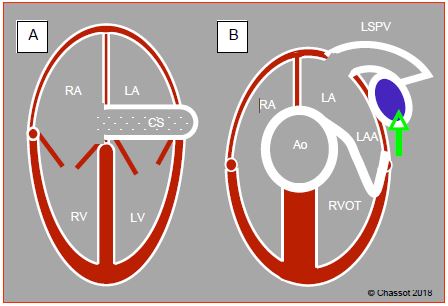
Figure 15.8: Left superior vena cava (LSVC). A: dilation of the coronary sinus (CS) (≥ 1.5 cm); injection of microbubbles into a vein in the left upper limb – these appear in the coronary sinus before reaching the RA. B: short-axis view of the LSVC which appears between the left superior pulmonary vein (LSPV) and the left atrial appendage (LAA).
Video: Dilatation of the coronary sinus in a case of left superior vena cava; the coronary sinus appears as a vertical tube near the top of the screen, draining into the right atrium; the right ventricle is hypertrophied.
The right superior vena cava (RSVC) may be present, hypoplastic or absent. Although LSVC is of no interest as a pathology, it entails risks for surgery or central venous canulation [2].

Figure 15.8: Left superior vena cava (LSVC). A: dilation of the coronary sinus (CS) (≥ 1.5 cm); injection of microbubbles into a vein in the left upper limb – these appear in the coronary sinus before reaching the RA. B: short-axis view of the LSVC which appears between the left superior pulmonary vein (LSPV) and the left atrial appendage (LAA).
- Thromboembolic risk linked to a left central venous catheter, which may prompt thrombosis of the coronary sinus – any catheter placed in the jugular or subclavian vein must be removed as soon as possible. Swan-Ganz catheters are contraindicated. If the RSVC is hypoplastic, a right-sided catheter may be virtually occlusive. If the RSVC is absent, a right jugular or subcalvian canulation can perforate the dead end. The problem is identical for endovenous pace-maker.
- Problem in terms of venous cannulation during CPB – the LSVC must be drained or excluded if the RSVC is of an appropriate gauge.
- In the event of retrograde cardioplegia, the infusate leaks into the SVC and does not perfuse the heart.
- Frequent associated AV blocks.
- In the event of drainage into the LA (coronary sinus-type ASD).
- In the event of intravenous pacing.
The fortuitous discovery of an enlarged coronary sinus at preoperative echocardiography is suspicious of LSVC; it requests a CT-scan to confirm the diagnosis and to precise the presence or absence of a RSVC [2]. A pulmonary vein may take an anomalous course to the RA instead of anastomosing normally with the LA. This usually concerns a right superior pulmonary vein which connects with the root of the SVC into the RA (Figure 15.9). This anomaly results in right-sided overload (L-to-R shunt) with dilation of the SVC or IVC (depending on the connection), RA, and RV. It is often associated with a sinus venosus-type ASD.
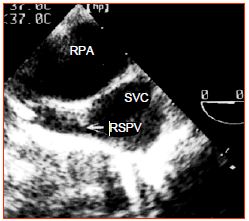
Figure 15.9: Partial anomalous pulmonary venous return. The right superior pulmonary vein (RSPV) flows into the superior vena cava (SVC) just above its anastomosis into the RA. RPA: right pulmonary artery (short-axis view of the ascending aorta at 0° with clockwise rotation of the probe).
Total anomalous pulmonary venous return constitutes a neonatal emergency (see Figure 14.33). It is not observed in adults since it is either operated on in childhood or results in death at an early age.
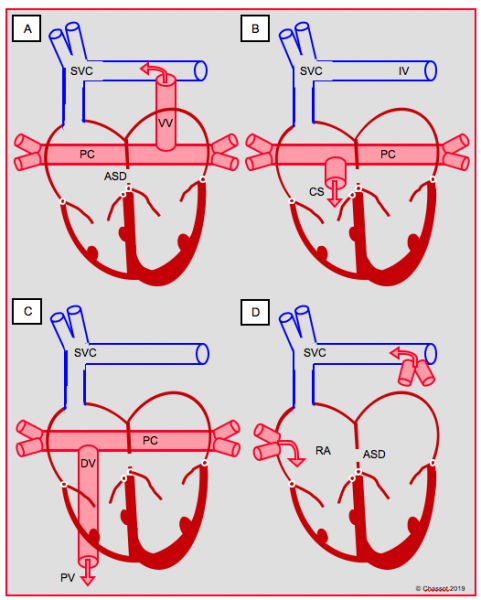
Figure 14.33: Diagram showing total anomalous pulmonary venous return. A: supracardiac type, into the innominate vein (IV) via a vertical vein (VV). B: cardiac type, via the coronary sinus (CS). C: infracardiac type, transdiaphragmatic into the portal vein (PV) via a descending vein (DV). D: mixed type. In the first three cases, a posterior collector (PC) drains venous blood from both lungs into a system located behind the atria.
Treatment involves reimplanting the anomalous pulmonary vein in the LA. Surgical correction of anomalous pulmonary venous return combined with a sinus venosus-type ASD involves creating a patch inside the anastomosis of the SVC into the RA so that the arterialised blood is diverted to the LA through the ASD (Warden procedure) [1]. Postoperatively, the flow should exhibit the systolic and diastolic biphasic morphology of central venous flows on an echocardiogram. The maximum velocity should remain below 1.0-1.5 m/s. A continuous non-oscillating flow with Vmax > 1.5 m/s indicates prohibitive restriction and requires surgical revision [4].
© BETTEX D, CHASSOT PG, January 2008, last update February 2020

Figure 15.9: Partial anomalous pulmonary venous return. The right superior pulmonary vein (RSPV) flows into the superior vena cava (SVC) just above its anastomosis into the RA. RPA: right pulmonary artery (short-axis view of the ascending aorta at 0° with clockwise rotation of the probe).
Total anomalous pulmonary venous return constitutes a neonatal emergency (see Figure 14.33). It is not observed in adults since it is either operated on in childhood or results in death at an early age.

Figure 14.33: Diagram showing total anomalous pulmonary venous return. A: supracardiac type, into the innominate vein (IV) via a vertical vein (VV). B: cardiac type, via the coronary sinus (CS). C: infracardiac type, transdiaphragmatic into the portal vein (PV) via a descending vein (DV). D: mixed type. In the first three cases, a posterior collector (PC) drains venous blood from both lungs into a system located behind the atria.
Treatment involves reimplanting the anomalous pulmonary vein in the LA. Surgical correction of anomalous pulmonary venous return combined with a sinus venosus-type ASD involves creating a patch inside the anastomosis of the SVC into the RA so that the arterialised blood is diverted to the LA through the ASD (Warden procedure) [1]. Postoperatively, the flow should exhibit the systolic and diastolic biphasic morphology of central venous flows on an echocardiogram. The maximum velocity should remain below 1.0-1.5 m/s. A continuous non-oscillating flow with Vmax > 1.5 m/s indicates prohibitive restriction and requires surgical revision [4].
| Anomalous venous returns |
|
Left superior vena cava – drains into the widened coronary sinus. Risks: thrombosis of the coronary sinus due to a central catheter, leakage of retrograde cardioplegia into the SVC.
Anomalous pulmonary venous return: in adults, only affects one pulmonary vein connected to the RA – equivalent to a L-to-R shunt. |
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
- AGGARWAL N, GADHINGLAJKAR S, SREEDHAR R, et al. Warden repair for superior sinus venosus atrial septal defect and anomalous pulmonary venous drainage in children: anesthesia and transesophageal echocardiography perspectives. Ann Card Anaesth 2016; 19:293-9
- SHOIAB I, SCHAFF H, SARAN N, et al. Recommendations for perioperative management in patients with absent right superior vena cava. J Cardiothorac Vasc Anesth 2019; 33:1710-3
- STÜMPER O, SUTHERLAND GR. Transesophageal echocardiography in congenital heart disease. London: Edward Arnold, 1994, 37-38
- VICK GW: Pulmonary venous and systemic ventricular inflow obstruction in patients with congenital heart disease: Detection by combined two-dimensional and Doppler echocardiography. J Am Coll Cardiol 1987; 9:580-4,
