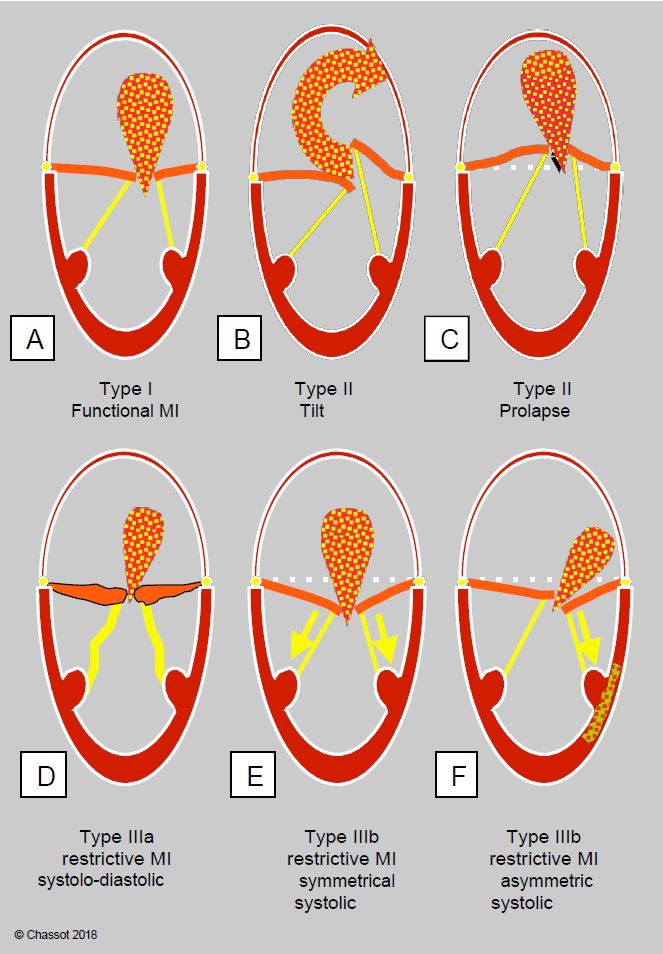
- Type I: normal leaflet motion and structure;
- Type II: excessive leaflet movement (tilting in the LA);
- Type III: restrictive leaflet movement:
- IIIa: systolic-diastolic restriction (rigid, poorly mobile leaflets);
- IIIb: systolic restriction only (retention of the leaflets in the LV).
Types II and IIIa are structural primary MIs, whereas types I and IIIb are functional secondary MIs.
Figure 11.67: Carpentier's classification of mechanisms of mitral regurgitation and morphology of the MI jet. A: Type I, normal leaflets. B: Type II, excess valve tissue with tilting of one leaflet (± chordal tear). C: Type II, excess leaflet tissue with coaptation point > 2 mm posterior to the annulus plane (long-axis view 120-140°). D: Type IIIa, systolic-diastolic leaflet regurgitation in the ARF. E: Type IIIb, symmetric systolic leaflet limitation with global LV dilatation. F: Type IIIb, asymmetric systolic leaflet restriction in LV ischaemia with segmental akinesia/dyskinesia.
Functional impairment (type I)
Functional regurgitation due to annular dilatation or calcification (MAC) preventing systolic contraction of the annulus; leaflet motion is normal. MI due to clefting of the septal leaflet in the atrioventricular canal or MI due to endocardial perforation are included in this category. Dilatation of the mitral annulus itself is due to several phenomena [6].
Video: Three-dimensional view of the mitral valve from the LA; presence of a cleft in the anterior leaflet.
La dilatation de l'anneau mitral est elle-même due à plusieurs phénomènes [6].
- Chronic dilatation of the LV due to ventricular dysfunction and failure or remodelling;
- Acute dilatation of the LV due to volume or pressure overload; of variable severity, MI may be transient;
- Basal kinesia;
- Massive dilatation of the LA.
Dilatation of the annulus occurs mainly in the posterolateral part where it is thinnest; in the anterior part the annulus is attached to the fibrous trine and the aortic valve and cannot change shape [3]. On echocardiography, this type of MI is characterised by a central regurgitant jet in the LA with circular symmetry that more or less crosses the atrial cavity (see Figure 11.26A). The ratio of the anteroposterior diameter of the annulus to the length of the anterior leaflet is > 1.3 (long-axis view 120-140°).
Video: Type I central mitral insufficiency; the MI is minor.
Video: Type I central mitral insufficiency; the MI is minor to moderate.
Le rapport entre le diamètre antéro-postérieur de l'anneau et la longueur du feuillet antérieur est > 1.3 (vue long-axe 120-140°).
Mitral prolapse (type II)
In mitral prolapse, the range of motion of one or both leaflets is increased and the valve has excess tissue. As mentioned above, there are several forms of this condition (Figure 26.13).
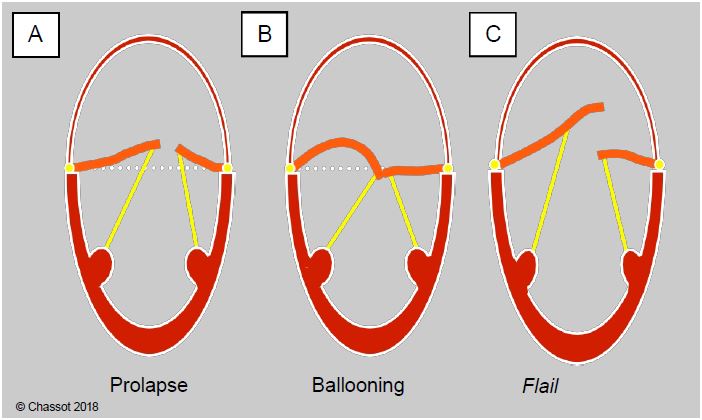
- Ballooning of a leaflet; part of the body of a leaflet protrudes into the LA, but the coaptation point is in a normal position, on the ventricular side of the plane of the mitral annulus;
- Coaptation point > 2 mm posterior to the plane of the mitral annulus (defined in the 120° long axis view of the LV, see Figure 11.3);
Video: Prolapse of the medial part of the posterior leaflet (P2) in 0° 4-cavity view.
- Flail leaflet in the LA;
Video: Degenerative prolapse with ballooning and remodelling of both mitral valve leaflets (2-cavity 90° view).
- A flail leaflet caused by stretching or breakage of the chord;
Video: Three-dimensional view from the LA of a prolapse of the anterior festoon of the posterior leaflet (P1) with chord rutpure, located on the screen on the lower left part of the valve.
Video: Prolapse and remodelling of the middle part of the posterior leaflet (P2, on the left of the screen) in 120° long-axis view; the regurgitation orifice is large.
Video: Prolapse and chord rupture of the middle and posterior festoons of the posterior leaflet (P2-P3) in 3D long-axis view of the LV.
- A combination of the above.
Figure 26.13: Distinction between prolapse (A), ballooning (B) and flail (C). In the first case, the coaptation point is located ≥ 2 mm posterior to the plane of the mitral annulus, in the LA. In the second case, the coaptation point is normally located, but excess leaflet tissue causes systolic bulging of the leaflet body. In tilting, all or part of a leaflet is tilted into the LA, most often with rupture of the chord(s).
Transoesophageal echocardiography is the test of choice to demonstrate these different lesions (see Figure 11.64). The appearance of the intra-atrial jet provides a key to the mechanism involved (Figure 11.26B) [12].
- Central in the case of simple retraction of the coaptation point;
Video: Type I central mitral insufficiency.
- Eccentric and oblique in the LA in the case of tilting or cord rupture; the jet is directed away from the affected leaflet: towards the interatrial septum in the case of posterior leaflet prolapse and towards the posterolateral wall in the case of anterior leaflet prolapse.
Video: Eccentric mitral insufficiency directed towards the interatrial septum, due to prolapse of the posterior leaflet (4-cavity 0° view).
Video: Eccentric mitral insufficiency directed towards the lateral wall of the LA, due to prolapse of the anterior commissure on ischaemia of the anterior papillary muscle.
Restrictive regurgitation (type III)
In restrictive mitral regurgitation, leaflet motion is restricted and closure is inadequate because the leaflets are held below the plane of the mitral annulus during systole by various mechanisms (Figure 11.68).
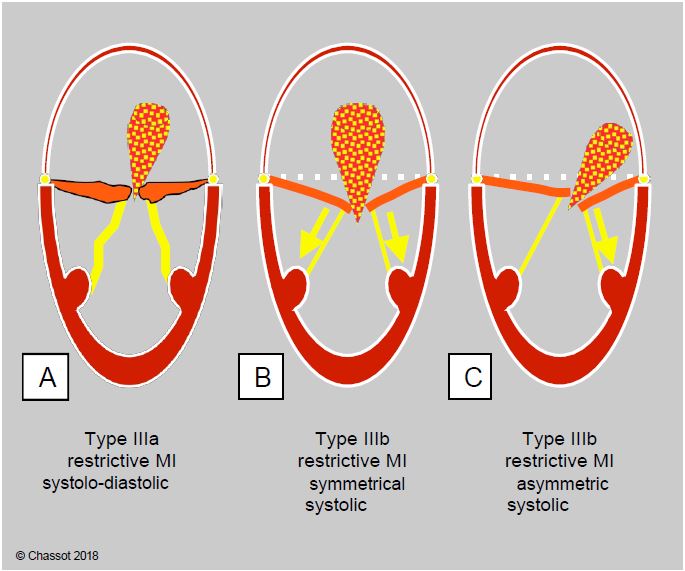
- Type IIIa (systolic-diastolic): the valvular and subvalvular apparatus are fibrosed and retracted; incomplete opening and closing of the valve is typical of rheumatic valve disease in which the leaflets are retained by commissural fusion.
Video: Commissural fusion in mitral stenosis.
- Symmetric type IIIb: Global dilatation of the ventricle spreads the chords, pulls on the chords and keeps the leaflets in the ventricular cavity during systole, below the coaptation plane. As the dysfunction and sphericity of the LV are homogeneous, the traction on the leaflets is symmetrical; the MI is central in the LA.
Video: Restrictive mitral insufficiency on dilatation and dysfunction of the LV; the mitral coaptation point is located well below the plane of the annulus.
Video: Restrictive mitral insufficiency on dilatation and dysfunction of the LV; the mitral leaflets remain almost immobile during the cardiac cycle.
- Asymmetric type IIIb: Although the same systolic traction mechanism is involved, segmental ischaemic dyskinesia affects only part of the ventricular wall and retracts only part of the leaflets. The MI is asymmetric and its jet is oblique in the LA.
Video: Restrictive mitral insufficiency due to severe anterolateral ischaemia.
Figure 11.68: Restrictive mitral regurgitation. A: Type IIIa, systolic-diastolic restriction of the leaflets in the ARF. B: Type IIIb, symmetric systolic restriction of the leaflets with global dilatation of the LV. C: Type IIIb, asymmetric systolic restriction of one leaflet in LV ischaemia with segmental akinesia/dyskinesia.
In left ventricular failure, restrictive MI (IIIb symmetric) is an excellent marker of the degree of ventricular dysfunction because it reflects the degree of LV dilatation; its increase during exercise is a good predictor of mortality [13]. On bypass it is a very sensitive indicator of momentary systolic failure (insufficient catecholaminergic support), volume overload (too swift weaning off bypass), excessive arterial resistance (excessive vasoconstrictor) or acute ischaemia (inoperative bypass).
Acute mitral insufficiency
Acute mitral insufficiency (MI) is caused by rupture of the leaflets (Barlow's disease), rupture of the cusps (ischaemic necrosis), endocarditis (perforation, tissue destruction) and acute LV failure (on weaning off ECC). There is no remodelling of the cavities in acute failure unless it occurs in the course of chronic disease. As the LA is not dilated, there is significant systolic reflux into the pulmonary veins (disappearance or inversion of the systolic component of pulmonary venous flow) and the "v" wave on the PCWP curve is massive. Left-sided congestion leads to pulmonary oedema, which can sometimes be unilateral if the MI jet is very oblique over the LA.
Medical treatment is aimed solely at stabilising the patient until surgery: vasodilators (nitroprusside), inotropic agents (dobutamine, milrinone ± adrenaline), positive pressure ventilation and intra-aortic balloon pump.
Remodelling
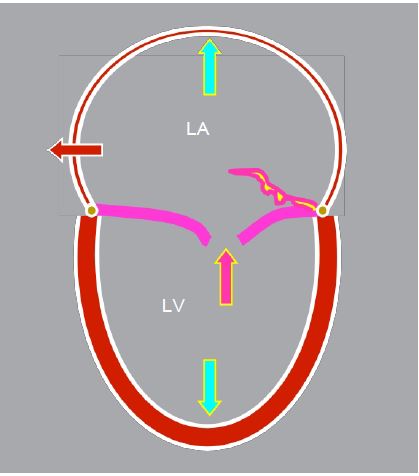
Mitral regurgitation leads to volume overload, with part of the ejected volume entering the LA and returning to the LV during the next diastole. This is associated with dilatation of the LA, an increase in ventricular end-diastolic volume (Vtd) and eccentric hypertrophy of the LV, proportional to the extent and duration of the disease (Figure 11.69). As the LV works under favourable conditions (low afterload and high preload), the ejection fraction is normal or high, masking the progressive dysfunction. By the time the patient becomes symptomatic, ventricular function is already significantly impaired, unlike in stenosis where symptoms occur before LV dysfunction. The more dilated the LA, the greater the risk of atrial fibrillation (AF). A prolapse with significant MI has a 51% rate of AF, ventricular failure and cardiac death at 5 years [4]. Left alone, this MI has a 90% mortality rate at 10 years (7-8%/year) [7].
Figure 11.69: Outline of the ventricles in severe mitral insufficiency; the LV and LA are dilated, the mitral leaflets do not coapitate or one leaflet tilts into the LA, the interatrial septum bulges into the RV in systole.
Pulmonary congestion and PHT are less common in mitral regurgitation than in stenosis. There are two reasons for this.
- Atrial dilatation increases the compliance of the LA, slowing the pressure wave caused by regurgitation.
- MI is systolic; the pressure increase is brief and does not last for the entire cardiac cycle. The mean PLA is much lower than the pressure at the peak of the 'v' wave; it is lower than that in the LA in mitral stenosis.
The absence of LV and LA dilatation is not compatible with severe chronic MI. However, it may be seen in acute MI.
MI and haemodynamics
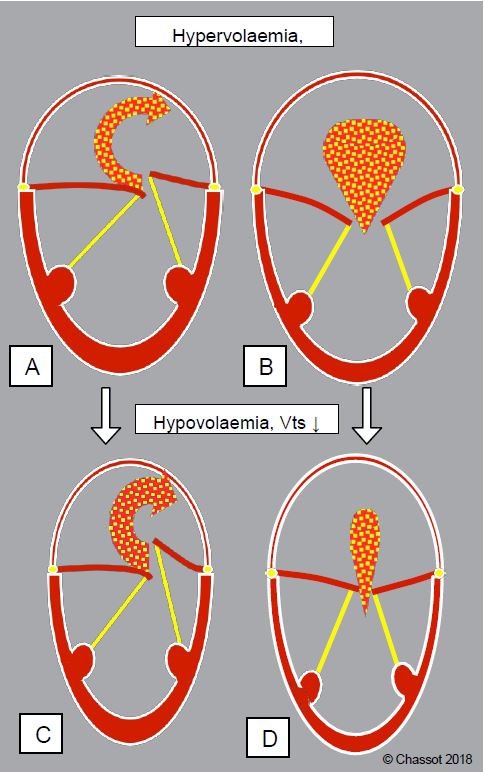
Although it is not uncommon to find situations where all three types of mitral regurgitation are combined, this classification is clinically important because the haemodynamic behaviour differs from one category to another (Figure 11.70):
Figure 11.70: Different behaviour of mitral regurgitation with variations in blood volume depending on the type of MI. A and B: hypervolaemia; the ventricle dilates, its telesystolic volume (Vts) increases; MI decreases in prolapse (A) because the chords are pulled downwards, but increases in types I and III (B) because the restriction of the mitral leaflets increases. C and D: Hypovolaemia; the ventricle becomes enlarged, its telesystolic volume decreases; MI increases in type II (C) because the chords have more travel, but decreases in types I and III (D) because the restriction decreases and the leaflets are almost able to coapt.
- Types I and III: Regurgitation is reduced by reducing ventricular volume in systole, allowing the leaflets to return to normal coaptation; this is achieved by reducing afterload, improving systolic function or reducing filling [11].
- Type II in prolapse (with retraction of the coaptation point): regurgitation is reduced by increasing the size of the ventricle in systole, which tightens the chords and brings the prolapsing leaflet towards the coaptation plane; this is achieved by increasing preload and decreasing contractility (see Barlow's disease). This phenomenon is significant in Barlow's disease associated with moderate or moderate to severe MI without chordal rupture. Reduced preload and afterload lead to an overestimation of the extent of the MI [5].
- Type II on chordal rupture: complete tilting of a chord or leaflet causes a severe MI, which is no longer modified by LV size, but is essentially dependent on systolic pressure: it increases in the event of a hypertensive surge [5].
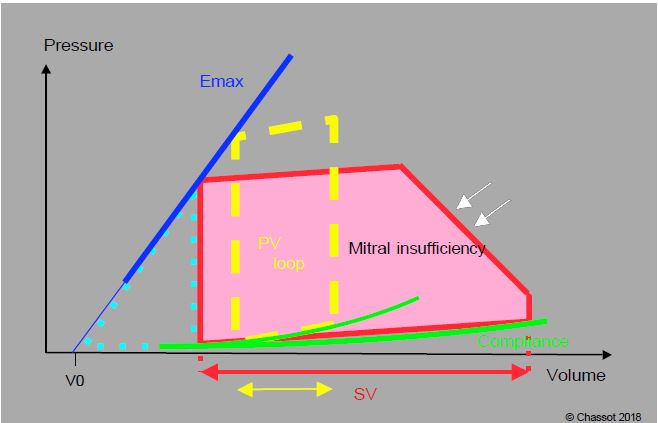
Whatever the mechanism, mitral regurgitation leads to volume overload of the left heart, since part of the systolic volume is only going back and forth between the atrium and the ventricle. The LV becomes hypertrophied and dilated, the orifice is enlarged and the geometry of the LV changes: it becomes more rounded because the sphere offers the maximum volume per unit of circumference. Volume overload is illustrated by the enlargement and rightward shift of the pressure-volume loop (Figure 11.71) (see Figure 11.19 for the construction of the PV loop). The leak into the LA maintains a constant low afterload and the isovolumetric contraction phase is virtually eliminated because regurgitation begins as soon as the intraventricular pressure exceeds that of the LA, well before the aortic valve opens. As a good volume pump, the left ventricle tolerates a regurgitant fraction of 40% and only deteriorates when this fraction exceeds 50% [9]. The P/V loop also shows the distribution of ventricular work between internal pressure work (Tpr) and external ejection work (Téj). In MI, most of the energy is devoted to external ejection work; pressure work is reduced because the failure acts as a valve to keep afterload low. Energy efficiency (Tpr/Tej ratio) is very good and O2 consumption is slightly increased, much less than in pressure overload. Compared to aortic insufficiency, afterload is lower and volume overload occurs at a lower pressure (PLA instead of PAdiast) [8]. However, the reduction in chronic afterload, while favouring ventricular ejection, also reduces long-term wall stress and decreases the contractile performance of myocardial fibres, which progressively involute [1]. The risk of ischaemia is only present in the presence of coronary artery disease.
Figure 11.71: Pressure-volume loop in chronic mitral regurgitation (MI). The slope of Emax and the compliance curve are normal, but the ejected volume (SV, red) is much higher than normal (yellow arrow). Since the mitral valve leaks as soon as the LV pressure rises, the isovolumetric contraction phase is virtually absent and the LV regurgitates into the LA before ejecting into the aorta, hence the oblique slope of this phase (white arrows). The work of pressure (light blue dotted triangle) is lower than normal. MI represents volume overload at low afterload, a situation that is energetically very favourable for the LV. In acute MI there is no ventricular dilatation, the volume is at the upper limit of normal, but the end-diastolic pressure is very high.
The regurgitant volume depends on the size of the mitral orifice in systole (> 0.4 cm2 in severe MI) and the pressure gradient between the LV and the LA. These two parameters are not fixed values: their variability is determined by a number of haemodynamic phenomena.
- Left ventricular afterload: The left ventricle functions as if it had two outlets, the aorta and the left atrium. Ejection impedance is obviously lower in the LV than in the arterial system; variations in peripheral arterial resistance will therefore determine the distribution of the two systolic flows. The volume of MI decreases as systemic resistance decreases. MI is extremely sensitive to systemic afterload, especially in functional MI.
- Preload is high because part of the stroke volume returns to the ventricle during the following diastole; anterograde flow through the mitral valve is high. Hypervolaemia dilates the ventricle and increases the size of the MI. However, the opposite is not true: hypovolemia is poorly tolerated even though it reduces ventricular volume. In fact, the regurgitant fraction increases as total systolic volume decreases because the impedance of the LA remains lower than that of the aorta, especially when hypovolaemia is accompanied by arterial vasoconstriction. The mitral leak acts as a constant escape phenomenon and systemic flow falls.
- Ventricular function is the main determinant of the pressure gradient between the LV and the LA. If contractility is low, MI flow is artificially reduced. Ejection fraction is a poor reflection of systolic function because much of the ejection takes place in the low pressure system of the LA; the LV is operating in a very favourable mode of high preload and low afterload, masking any fall in contractility.
- The compliance of the receiving cavity influences the degree of regurgitation. That of the LA depends on its volume and the tone of its wall. In chronic MI, the atrium dilates and becomes very thin, which favours regurgitation and buffers the pressure wave. Together with ventricular function, atrial compliance is the main determinant of the "v" wave generated by MI on the PCWP curve. The amplitude of this pressure wave does not reflect the degree of mitral regurgitation but the resistance of the LA to the flow of blood regurgitated in systole; it is very pronounced in acute MI because the atrium is of normal size and compliance, but moderate or normal in long-term MI when the LA is distended and very compliant (LA diameter > 5.5 cm) [10].
- Mean LA pressure: the onset of MI is delayed when this pressure increases. Positive pressure ventilation increases pulmonary venous return and slows down MI; in addition, the increase in endothoracic pressure reduces left ventricular transmural pressure and effective LV afterload. Patients with MI therefore tolerate positive pressure ventilation well.
Pulmonary venous pressure gradually increases during the course of the disease and pulmonary interstitial stasis develops. In addition to this increase in post-capillary pulmonary pressure, there is a reactionary increase in precapillary PAR, which contributes to pulmonary hypertension. However, right heart dilatation and decompensation are less common in mitral insufficiency than in stenosis.
| Pathophysiology of mitral insufficiency |
| Type I MI: functional insufficiency, normal leaflet movement and structure:
- Ring dilatation (dilatation of the LV) - Non-contraction of the annulus (calcifications, basal ischaemia) Type II MI: excessive movement of the leaflets, tilted into the LA during systole: - Prolapse (retreat of the coaptation point > 2 mm behind the plane of the long-view ring LV axis 120°) - Ballooning, excess tissue and floating leaflets in the LA - Sheet tilting, chord or pillar rupture Type III MI: leaflets retained on the ventricular side by excessive traction on the chords: - IIIa (systolo-diastolic restriction): retraction of the leaflets and chords (ARF) - IIIb symmetrical (systolic): traction on the chords by apical and lateral displacement of the papillary muscles (dilation of the LV) - IIIb asymmetric (systolic): akinesia/dyskinesia of segments adjacent to a pillar (ischaemia) Haemodynamic conditions in MI: - Low afterload (leakage into the RA begins during isovolumetric contraction) - High preload (regurgitated volume returns to the LV during the next diastole) - LV systolic function facilitated despite reduced contractility (non-discriminatory EF) - Ventricular dysfunction already present when the patient becomes symptomatic - High LA compliance if LA dilated (pressure damping, moderate "v" wave) - PLA (high, delays the onset of MI and reduces the ΔP LV - LA) The extent of MI depends on haemodynamic conditions: - IM ↑ if : RAS ↑ , Vts LV ↑ - IM ↓ if : RAS ↓ , Vts VG↓ , contractility ↑ - Type II MI: ↑ if LV vts ↓ |
| Haemodynamic characteristics of mitral insufficiency |
| High pre-load (LV volume overload)
Low afterload (MI leaks as soon as PLV > PLA) LV dilatation (end-diastolic diameter > 7 cm or > 4 cm/m2 ) Impaired systolic function despite preserved EF Criterion for LV dysfunction: telesystolic diameter > 4 cm (> 2.5 cm/m2 ) Functional MI increases if : SAR ↑ , Vtd ↑ , contractility ↓ Systemic CO depends on low SAR Intolerance to hypovolaemia |
References
- CARABELLO BA, NOLAN SP, McGUIRE LB. Assessment of preoperative left ventricular function in patients with mitral regurgitation: Value of the end-systolic wall stress - end-systolic volume ratio. Circulation 1981; 64:1212-7
- CARPENTIER A, CHAUVAUD S, FABIANI JN, et al. Reconstructive surgery of mitral valve incompetence. J Thorac Cardiovasc Surg 1980; 79:338-48
- GLASSON JR, KOMEDA M, DAUGHTERS GT, et al. Three-dimensional dynamics of the canine mitral annulus during ischemic mitral regurgitation. Ann Thorac Surg 1996; 62:1059-68
- GRIGIONI F, TRIBOUILLOY C, AVIERINOS JF, et al. Outcomes in mitral regurgitation due to flail leaflets: a multicenter European study. JACC Cardiovasc Imaging 2008; 1:133-41
- LANCELLOTTI P, FATTOUCH K, LA CANNA G. Therapeutic decision-making for patients with fluctuating mitral regurgitation. Nat Rev Cardiol 2015; 12:212-9
- LANCELLOTTI P, MOURA L, AGRICOLA E, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010; 11:307-32
- LING LH, ENRIQUEZ-SARANO M, SEWARD JB, et al. Clinical outcome of mitral regurgitation due to flail leaflet. N Engl J Med 1996; 335:1417-23.
- NWASOKWA O, CAMESAS A, WEG I, et al. Differences in left ventricular adaptation to chronic mitral and aortic regurgitation. Chest 1989; 95:106-11
- OH JK, SEWARD JB, TAJIK AJ. The echo manual. 3rd edition. Philadelphia, Lippincott Williams & Wilkins, 2006, 189-242.
- PU M, GRIFFIN BP, VANDERVOORT PM, et al. The value of assessing pulmonary venous flow velocity for predicting severity of mitral regurgitation: A quantitative assessment integrating left ventricular function. J Am Soc Echocardiogr 1999; 12:736-43
- STEVENSON LW, BRUNKEN RC, BELIL D, et al. Afterload reduction with vasodilators and diuretics decreases mitral regurgitation during upright exercise in advanced heart failure. J Am Coll Cardiol 1990; 15:174-9
- STEWART WJ, CURRIE PJ, SALCEDO EE, et al. Evaluation of mitral leaflet motion by echocardiography and jet direction by Doppler color flow mapping to determine the mechanism of mitral regurgitation. J Am Coll Cardiol 1992; 20:1353-61
- YIU SF, ENRIQUEZ-SARANO M, TRIBOUILLOY C, et al. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation 2000; 102:1400-6