Nosology
A combination of valvular heart disease is not uncommon. Almost 15% of valve surgery patients have another moderate or severe valve disease. Severe tricuspid regurgitation is present in 27% of mitral or aortic valve surgery cases. The most common combination of left-sided lesions is aortic stenosis combined with mitral insufficiency (20% of cases). The aetiology of multiple lesions is generally rheumatic (51% of cases) or degenerative (41% of cases); more rarely, the origin is related to endocarditis, radiation or the toxicity of certain substances (drugs, anorectics) [3].
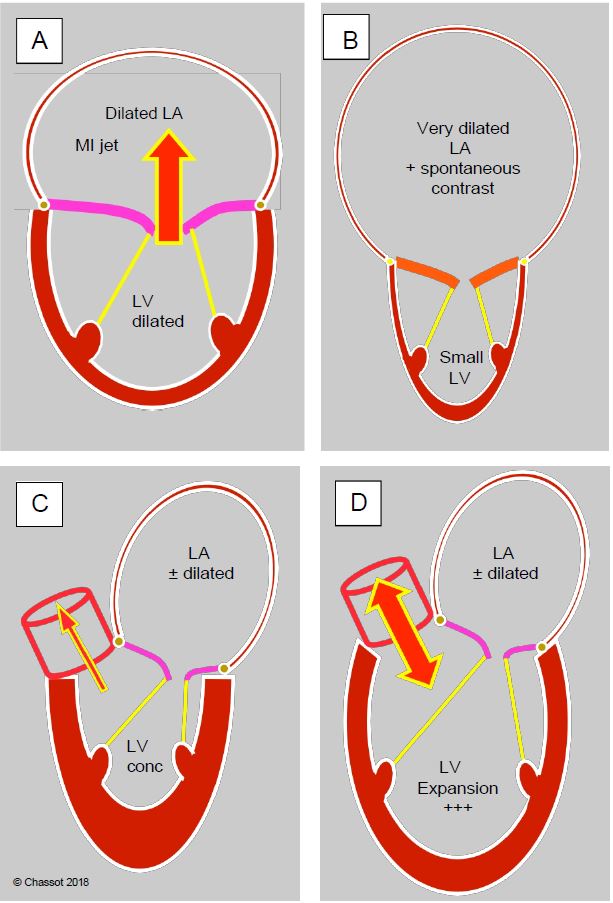
The complex involvement of several valves is the area par excellence where recipes no longer apply. The first step is to determine which pathology is the most restrictive, and this is not always clear from the preoperative studies. Intraoperative transesophageal echocardiography (TEE) often provides the answer, but unfortunately after induction (see Figure 11.164).
Video: Polyvalvulopathy on ARF; rheumatic disease has led to mitral disease (stenosis and insufficiency) and aortic insufficiency; as the LV is small and the LA very dilated, mitral stenosis is the predominant feature, and insufficiencies are secondary.
Management is then guided by the pathophysiology of the dominant disease, which will also determine the patient's exit from ECC and the immediate postoperative period. In general, stenosing lesions are more restrictive than insufficiencies. In addition, clinical symptoms are dominated by the most proximal lesion following the blood flow, for example mitral stenosis in combined mitral and aortic valve disease [2]. Finally, the degree of severity of each valvular insufficiency defines its importance within the combination of valvular insufficiencies.
Figure 11.164: In valvular heart disease, the silhouette of the ventricles shows the remodelling of the left cavities in response to haemodynamic stresses and helps to determine the dominant pathology. A: Mitral regurgitation; dilatation of the LV and LA. B: Mitral stenosis; small LV and huge LA. C: Aortic stenosis; concentric LVH and dilatation of the LA. D: Aortic insufficiency; massive dilatation of the LV, moderate dilatation of the LA.
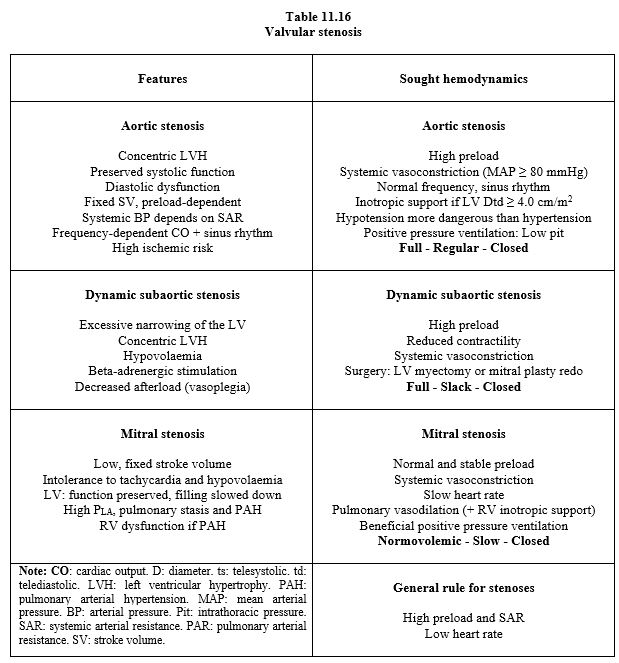
Tables 11.16 and 11. 17 summarise the recommended approach in isolated valvulopathies; they can be used as a basis for clinical management depending on the dominant lesion, but it is difficult to make recommendations in polyvalvulopathies because each lesion influences the others by exacerbating or masking them: For example, mitral stenosis reduces LV filling and stroke volume, thereby reducing the gradient across the aortic stenosis and the haemodynamic severity of the latter; conversely, mitral insufficiency (MI) further exacerbates ventricular dilatation due to aortic insufficiency (AI) [6]. Each case must be analysed individually. Certain combinations of valvular diseases may benefit ventricular function by limiting cavity remodelling or by counteracting each other: for example, MI reduces LV afterload while aortic stenosis increases it; although anterograde flow is restricted, ejection indices are improved.
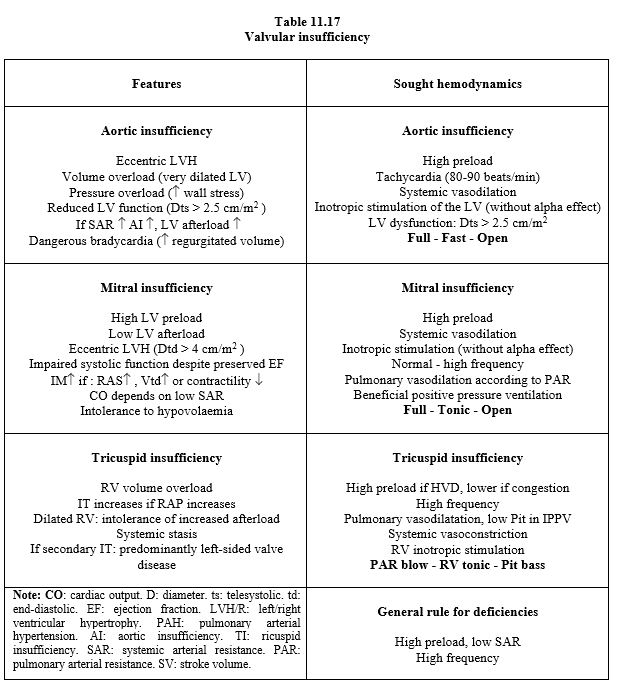
Mild or moderate mitral and tricuspid insufficiency are often functional disturbances secondary to pressure and/or volume overload that resolve when the main lesion is corrected.
Paraclinical evaluation
Even more so than in isolated valve disease, quantification of systolic function is a poor reflection of true myocardial performance and must be interpreted in the context of the disease. Ejection fraction is particularly poor for assessing LV systolic performance, especially in the presence of an MI that facilitates ejection. The dimensions of the ventricle are a better criterion of its contractile capacity. The values beyond which its function is reduced are [1,4,5]:
- Telesystolic diameter (short axis) ≥ 2.5 cm/m2 if failure predominates,
- Telesystolic diameter (short axis) ≥ 4.0 cm/m2 if stenosis predominates.
Echocardiographic measurements should be based on indices that are as independent as possible of loading conditions, such as 3D valve orifice planimetry, regurgitant or stenotic orifice area and vena contracta diameter [6]. Upstream stenosis decreases flow through the downstream valve and reduces its transvalvular gradient (underestimation of stenosis), whereas upstream insufficiency increases flow and leads to an overestimation of the degree of stenosis in the downstream valve. The continuity equation cannot be used to quantify mitral area in the presence of AI because the flow through the mitral valve is not the same as that through the aortic valve. Half-pressure time is invalid for quantifying mitral stenosis in the presence of severe aortic disease.
Pulmonary thermodilution (Swan-Ganz catheter) is invalid in severe tricuspid regurgitation and does not reflect left-sided flow in mitral pathology. PiCCO measures effective peripheral systemic flow but not LV stroke volume in mitral or aortic insufficiency.
| Polyvalvulopathy |
|
The combination of multiple valvular pathologies creates haemodynamic situations that vary widely from patient to patient. Here are some general remarks:
- Stenosis is more restrictive than insufficiency
- Upstream valve disease takes precedence over downstream valve disease
- Whatever the combination, one pathology predominates and controls haemodynamics.
- Ventricular remodelling gives a good idea of the dominant lesion
- LV function is best assessed by its telesystolic and telediastolic dimensions.
- TEE assessment needs to be based on measurements that are less dependent on haemodynamics (plano 3D stenosis metrics, vena contracta, regurgitation area).
|
References
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BONOW RO, BRAUNWALD E. Valvular heart disease. In: ZIPES DP, et al, eds. Braunwald’s heart disease. A textbook of cardiovascular medicine. 7th edition. Philadelphie: Elsevier Saunders, 2005, 1553-632
- IUNG B, BARON G, BUTCHART EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231-43
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2017; 70:252-89
- UNGER P, CLAVEL MA, LINDMAN BR, et al. Pathophysiology and management of multivalvular disease. Nat Rev Cardiol 2016; 13:429-40