Kidneys receive 20% of cardiac output, but consume only 10% of transported oxygen. The glomeruli passively filter plasma; they receive the majority of the renal plasma flow (RPF), but have a low O2 intake compared to the medulla. The medulla, on the other hand, has a 5-10 times lower blood flow, but has a high O2 intake as it does actively concentrate urine; it is therefore much more sensitive to ischaemia and hypovolaemia [6]. Glomerular filtration rate (GFR) is directly proportional to intravascular hydrostatic pressure and inversely proportional to oncotic pressure (normally 25 mmHg). GFR decreases with age from 125 mL/min in young adults to 80 mL/min at 60 years and 60 mL/min at 80 years [59].
If blood pressure falls, renal selfregulation maintains RPF and glomerular filtration by vasodilation of preglomerular arterioles (myogenic mechanism) and by vasoconstriction of postglomerular arterioles (angiotensin II). When renal perfusion decreases by more than 50% during 1 hour, GFR decreases proportionally much more than FPR; FPR is then preferentially distributed towards the deep cortical zone and the medullary. A decrease in cardiac output and perfusion pressure leads to sympathetic stimulation, vasopressin and ADH secretion, and activation of the renin-angiotensin system, all of which contribute to the retention of water and sodium by kidney [2]. Hemodilution in bypass surgery increases renal plasma flow and decreases intraparenchymal vascular resistance, but decreases O2 . Hypothermia and non-pulsatility induce preferentially cortical vasoconstriction.
Normally, renal function is not significantly altered by either anaesthesia, surgery or ECC; standard tests such as creatinine levels or clearance are minimally altered in the majority of cases, but they are neither sensitive nor early enough to trigger treatment before functional damage develops. However, subtle changes can be detected by finer measurements such as biomarkers like NGAL, KIM-1 or cystatin C [16,18]; the change in creatinine clearance during protein overload, which measures renal functional reserve, decreases for several weeks after bypass surgery [41]. However, the predictive value of these tests for the development of acute kidney disease is modest [23]. On the other hand, this minor dysfunction may amplify the impact of a pre-existing but sub-clinical functional impairment (arterial disease, age, etc) or be potentiated by intraoperative events: hypotension, nephrotoxic drugs, etc. Studies comparing the way pH is adjusted, hypothermia versus normothermia, pulsatile versus non-pulsatile flow, have so far not been able to demonstrate a significant advantage of one bypass technique over another in preserving renal function [53].
However, perfusion pressure and O supply2 (DO2 ) are for sure key factors. It has long been demonstrated that in one third of cases, a change in postoperative creatinine is dependent on MAP during bypass surgery, provided that pump output and circulating volume are normal [62]. The renal DO2 is crucial for the functional survival of kidney. However, by virtue of vasoconstriction and haemodilution, the renal parenchymal O2 extraction increases by 40%. These phenomena continue during the first postoperative hours [33]. They are not corrected by the administration of crystalloid or colloid to maintain normovolaemia [56]. It is therefore not surprising that haemodilution is a risk factor for renal dysfunction; Ht should remain ≥ 26-28% [49].
Urine output is usually lowered during bypass surgery, but is a poor predictor of acute kidney disease. A value of < 0.5 mL/kg/h during ECC is generally considered predictive of postoperative dysfunction, but is related to many confounding preoperative factors [12]. Only a flow rate > 1.5 mL/kg/h ensures that renal function is normal in the postoperative period. The association between low urine output and secondary renal failure is strongest at low blood pressure (MAP < 80 mmHg) [24].
Post-operative renal failure
The currently popular revised version of the RIFLE and AKIN classifications is the KDIGO (Kidney Disease Improving Global Outcomes) nomenclature: postoperative renal failure is subdivided into four grades of severity based on serum creatinine level and urine output [5,32].
- Stage I: creatinine increased by 25m mol/L or 1.5-1.9 times baseline, urine output < 0.5 mL/kg/h for 6-12 hours.
- Stage II: creatinine increased 2-2.9 times baseline, urine output < 0.5 mL/kg/h for > 12 hours.
- Stage III: increase in creatinine to ≥ 350 mcmol/L or > 3 times baseline, urine output < 0.3 mL/kg/h for > 24 hours or anuria for > 12 hours, or haemodialysis/haemofiltration.
- Stage IV: long-term anuria (>4 weeks) requiring permanent dialysis.
This clinical deterioration is preceded by subclinical tissue damage characterised by elevated biomarkers such as NGAL or IGFBP7 (see Chapter 23, Renal Complications) [25].
The incidence of renal failure (stages III and IV) after cardiac surgery in ECC is 3-6% overall; simple renal dysfunction (stages I and II) is more common: 15-22% [15,25,26]. It depends on the type of operation: 7.7% after LV surgery, 3.9% after valve surgery, and 0.5% after CABG. When dialysis is required, mortality is high: 30% in adults and 45% in children [15,43]. Transient postoperative dysfunction (creatinine 150-250 mcmol/L, 20-25% increase) is more common: it occurs in 11-22% of patients and resolves within days or weeks [13,54]. A 25-50% decrease in glomerular filtration rate is present in 24% of patients after cardiac surgery (see Chapter 23, Renal Complications). The origin of renal failure that may occur after bypass surgery is multifactorial. Amongst the elements involved are, in probable order of importance [15,17,18,58,63].
- Preoperative clinical status:
- Preoperative nephropathy (creatinine ≥ 200 mcmol/L); primary renal disease, or secondary to diabetes, hypertension or polyvasculopathy.
- Left ventricular dysfunction (EF < 0.35), intra-aortic counterpulsation.
- Age of the patient (> 65 years); glomerular filtration normally decreases from 125 mL/min in the young to 80 mL/min at 60 years and < 60 mL/min at 80 years (loss of renal reserve of 0.75 mL/1.75 m2 per year from 30 years).
- Comorbidities: diabetes, hypertension, arterial disease, COPD.
- Decreased renal plasma flow leading to tissue hypoxia:
- Hypovolaemia and systemic hypotension (MAP < 30% of normal for more than 10 minutes).
- Low flow rate in bypass surgery (< 1.8 L/min/m2 ) and postoperatively (CI < 2 L/min/m2 ).
- Systemic venous congestion (CVP > 12 mmHg).
- Renal congestion on hypervolaemia (renal compartment syndrome).
- Use of alpha arterial vasoconstrictors (except terlipressin).
- Septic condition.
- In 75% of cases, acute nephropathy is related to sepsis and/or circulatory collapse.
- Effects of surgery:
- Complex operation, recovery.
- Aortic clamping versus beating heart surgery or stenting.
- Clamping of the descending aorta, ischaemia-reperfusion injury.
- Embolisation of atheromas or particles (catheterisation, aortic manipulation).
- Emergency operation or re-operation.
- Effects of ECC:
- Duration of ECC and aortic clamping, depth of hypothermia; bypass surgery decreases O2 supply to the renal parenchyma by 20% [33].
- Systemic inflammatory response (free radicals, cytokines, etc) and endotoxins [55].
- Anemia (haemodilution at Ht ≤ 26%); an Hb value < 100 g/L doubles the risk of acute kidney disease.
- Red blood cell transfusions (> 2 units).
- Haemolysis (haemoglobinuria) and rhabdomyolysis (myoglobinuria).
- Tubulotoxicity of free Fe2+ generated by haemolysis and oxidative stress (ischaemia-reperfusion, free radicals) [20].
- Use of nephrotoxic substances:
- X-ray contrast media (coronary angiography, angio-CT within 5 days preoperatively).
- Non-steroidal anti-inflammatory drugs (NSAIDs).
- Converting enzyme and angiotensin receptor blockers, metformin.
- Aminoglycoside antibiotics.
- Calcineurin inhibitors (tacrolimus, cyclosporine).
- Starch-derived colloids (HES) and aprotinin (discontinued substances) [29,38].
- Genetic polymorphism identifying individual risk of postoperative renal failure [57].
| Post-ECC renal failure |
| Etiological factors :
- Preoperative nephropathy (creatinine > 200 mcmol/L), the most important factor
- Hypovolaemia and hypotension (MAP < 70% of its usual value for > 20 minutes)
- Low ECC flow (< 1.8 L/min/m2 )
- Insufficient O2 intake (Ht < 26%)
- Long hypothermic bypass surgery
- Age > 65 years
- Nephrotoxic substances
- SIRS, haemolysis, rhabdomyolysis
|
Kidney protection
Optimisation of haemodynamics intra- and postoperatively (MAP > 75 mmHg, DC > 2.5 L/min/m2 , minimum Ht in ECC > 26%, DO2 > 600 mL/min/m2 , SvO2 > 70%) with preservation of circulating volume and inotropes significantly reduces the incidence of nephropathy (OR 0.64) and mortality (OR 0.66) [7]. Fluid replacement with strict hemodynamic monitoring (stroke volume, cardiac output, SvO2 ) provides a better match between infusions and needs, both in volume and timing (see Chapter 4 Fluid requirements); it significantly decreases the incidence of acute postoperative nephropathy, especially in high-risk patients [7].
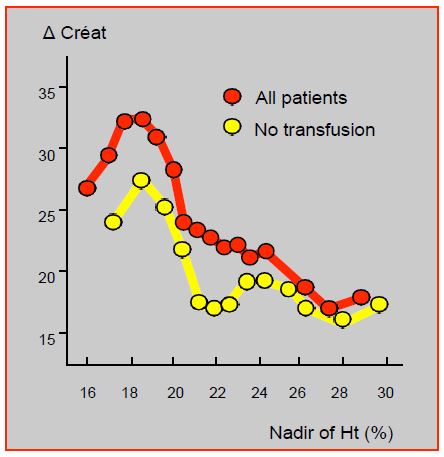
Anemia, transfusions and redo for haemostasis are three of the most important predictors of renal failure (OR 1.8-2.9), but are also among the most under control [30]. Postoperative renal function and renal failure rates worsen linearly as Ht falls below 30%; this worsening is maximal when the lowest Ht is below 24% [35]. The incidence of acute kidney disease is 4.1% in anaemic patients and 1.6% in eucythemic patients [31]. Mortality increases in direct relation to the extent of renal injury. However, for a similar Ht value, transfused patients systematically show a worsening of their renal function compared with those who are not transfused; their mortality is higher (3.8% versus 1.4%) and their incidence of renal failure is greater (12% versus 3.4%) [22]. This brings out a troubling dilemma: anaemia worsens the situation, but transfusion, rather than correcting it, adds a further deleterious factor (Figure 7.27). Which requires an even more imperative anaemia correction preoperatively.
Figure 7.27: Change in postoperative creatinine as a function of lowest Ht. Post-operative renal function and renal failure rate worsen linearly when the lowest Ht is below 24%. However, for a similar Ht, patients who are transfused systematically show a worsening of their renal function compared to those who are not transfused [22].
Some substances are believed by anaesthetists to be "protective" of renal function. However, few of these substances appear to have a beneficial effect on postoperative renal function (see Chapter 24, Renal protection) [47].
- Mannitol is completely filtered in the glomeruli, and is not resorbed in the tubules: it increases plasma volume and diuresis by increasing water excretion and decreasing sodium reabsorption; moreover, it has antioxidant activity and opposes the effects of hydroxyl groups ("free radicals") released during revascularisation. There is very little evidence that it is effective as a protective agent against the effects of renal ischaemia; no clinical study has shown any improvement in the prognosis of postoperative renal failure [52]. At 0.5 g/kg in the ECC priming fluid, it increases blood volume, glomerular capillary pressure, proximal tubular pressure and diuresis, but no beneficial effect on perioperative renal function could be demonstrated [27]. It is probably only effective in cases of haemolysis or rhabdomyolysis.
- Furosemide increases RPF and lowers renal vascular resistance in addition to its diuretic effect. Its prophylactic use clearly decreases the incidence of anuria, but only modifies the prognosis of renal failure in the sense of a non-significant increase in the need for dialysis [9,34]. Intraoperatively, its only use is to reduce excess interstitial fluid after ECC (10-20 mg) when the water balance is too positive as evidenced by the presence of hypoxaemia not responding to 100% O2 ventilation with PEEP. It is also useful in monitoring the recovery of renal function after renal-vascular reconstruction or ischaemia and reperfusion. High doses help to remove free haemoglobin or myoglobin.
- Dopamine at 1-3 mcg/kg/min increases RPF, glomerular filtration rate and diuresis, but has not been associated with postoperative renal function or outcome in critically ill patients [4]. Although it has a positive effect on diuresis, no conclusive clinical study has shown any benefit of prophylactic use of dopamine in postoperative renal failure, whether preoperative function is normal or impaired [39,46]. It does not increase cortical renal plasma flow in ECC, nor does it compensate for the decrease in cortical perfusion secondary to the drop in perfusion pressure below 70 mmHg [37]. As the rate of postoperative arrhythmias is higher when associated with dopamine, the value of this prophylaxis remains unjustified [14]. In addition, it may be responsible for post-ECC proximal tubular dysfunction [11].
- Fenoldopam is a dopamine analogue that selectively stimulates DA1 receptors and induces a dose-dependent increase in RPF and diuresis (0.03-0.3 mcg/kg/min) [8]. It has the advantage of increasing RPF in the cortex and medulla. It does not cause tachycardia or arrhythmias, but has a marked hypotensive effect as it is a potent arteriolar vasodilator. The nephro-protective activity of continuous infusion (0.1 mcg/kg/min) has been demonstrated in recent studies, but is not confirmed by all studies [48,61].
- Levosimendan is the only inotropic substance associated with a reduced risk of acute kidney disease [60].
- Statins; the data are contradictory. Some studies tend to show a lower incidence of acute nephropathy in patients on statins before and after surgery [36,44], but the most recent study found no benefit to rosuvastatin [65]. Like aspirin, statins reduce postoperative mortality, but probably have no effect on the incidence of nephropathy [10].
- Halogen preconditioning: sevoflurane use during ECC reduces markers of postoperative renal dysfunction such as cystatin C [28]. Remote ischaemic preconditioning (see Myocardial protection) tends to decrease the incidence of post-ECC nephropathy [64].
- Antioxidants such as N-acetylcysteine (Salmucol® ) or allopurinol (Xyloric® ) reduce inflammatory markers and block the toxic effect of free radicals, but their clinical use has been disappointing and does not provide any benefit in terms of mortality or morbidity in aortic surgery or cardiac surgery with ECC [51].
- Two substances with anti-inflammatory and anti-apoptosis effects, minocycline (tetracycline) and EPO, appear to significantly reduce the proportion of patients developing acute kidney disease after cardiac surgery [19]. Steroids have questionable efficency [1].
- The administration of bicarbonate to alkalinise the urine appears to be able to alleviate postoperative nephropathy, in particular by solubilising free Hb related to ECC haemolysis and by activating the chelation of free Fe2+ [20,21].
While the impact of pharmacological prophylaxis is variable and disputed, there are a number of technical measures in patient management that can reduce the incidence and severity of acute kidney disease [3,42].
- At least 5 days between contrast examinations and surgery; overhydration to force diuresis has no significant benefit.
- Perioperative use of aspirin and statins.
- NSAIDs stopped 3 days before surgery, and ACE inhibitors stopped for 24-48 hours.
- Avoid all nephrotoxic agents (aminoglycosides, vancomycin, etc.) or those that modify renal plasma flow (ACE inhibitors, ARBs).
- Preoperative anaemia should be avoided (preparation with iron and/or EPO) [31].
- Titration of crystalloid infusates to maintain blood volume and haemodynamics as close to normal as possible:
- MAP ≥ 75 mmHg ;
- PCWP 12-15 mmHg, CVP < 10 mmHg;
- Urine output > 0.5 mL/kg/h;
- Minimum Ht in ECC > 26%.
- Prefer crystalloids over HES or colloids, and buffered solutions (Hartmann, Ringer, Plasmalyte) over NaCl [40,42]. Albumin is theoretically the best plasma expander, but it does not reduce the incidence of NPA [6]; it is only indicated if plasma levels are < 40 g/L [40].
- Prefer less invasive techniques such as stenting or beating heart surgery; beating heart coronary artery bypass grafting (OPCAB) reduces the incidence of APN in high-risk patients (OR 0.6) but probably not in those with normal renal function [45].
- Limit the duration of ECC.
- Limit intraoperative bleeding, avoid excessive haemodilution (Ht min 26%) and restrict blood transfusion.
- Administration of Na bicarbonate+ and possibly mannitol in case of haemolysis (dark red urine).
There are five elements that play a major role in the genesis of acute postoperative nephropathy and are relatively easy to manage when present during ECC:
- Duration of ischaemia (clamp time);
- Low pump output;
- Hemodilution;
- Hypovolaemia;
- Low blood pressure.
The first two are the responsibility of the operator and the perfusionist, but the other three are in the hands of the anaesthetist and intensivist. The maintenance of normal circulating volume, adequate O2 transport, adequate perfusion pressure and satisfactory pump output is therefore the key to renal prevention. However, the relationship between arterial hypotension in bypass surgery and the incidence of acute postoperative nephropathy is far from clear. It becomes less clear when all contributing factors that are known causes of renal failure are excluded [50].
| Kidney protection |
|
Overriding factors :
- Normovolemia
- Normotension (MAP 80 mmHg, minimum 70 mmHg on bypass)
- DC ≥ 2.4 L/min/m2 in normothermia, 1.8 L/min/m2 in hypothermia (ECC)
- DO2 normal: minimal Ht > 26% without transfusions
- Absence of nephrotoxic agents
Possible pharmacological protection: fenoldopam, bicarbonate, statins
Most suitable inotropic agents: dobutamine, milrinone, levosimendan
No protective effect (although urine output): dopamine, mannitol, diuretics
The best protection is to maintain normal MAP (≥ 75 mmHg), blood volume and Hb, while avoiding excessive alpha vasoconstrictors and transfusions.
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- AUGOUSTIDES JGT. The inflammatory response to cardiac surgery with cardiopulmonary bypass: shoud steroid prophylaxis be routine? J Cardiothorac Vasc Anesth 2012; 26 :952-8
- ARONSON S, BLUMENTHAL R. Perioperative renal dysfunction and cardiovascular anesthesia: concerns and controversies. J Cardiothor Vasc Anesth 1998; 12:567-86
- ARORA P, KOLLI H, NAINANI N, et al. Preventable risk factors for acute kidney injury in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2012; 26:687-97
- BELLOMO R, CHAPMAN M, FINFER S, et al. Low-dose dopamine in patients with early renal dysfunction: A placebo controlled randomized trial. Lancet 2000; 356:2139-43
- BIRNIE K, VERHEYDEN V, PAGANO D, et al. Predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care 2014; 18:606
- BREZIS M, ROSEN S. Hypoxia of the renal medulla - its implications for disease. N Engl J Med 1995; 332:647-55
- BRIENZA N, GIGLIO MT, MARUCCI M, FIORE T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med 2009; 37: 2079-90
- BROGDEN RN, MARKHAM A. Fenoldopam: a review of its pharmacodynamic and pharmacokinetics properties and intravenous clinical potential in the management of hypertensive urgencies and emergencies. Drugs 1997; 54:634-50
- BROWN CB, OGG CS, CAMERON JS. High-dose furosemide in acute renal failure: A controlled clinical trial. Clin Nephrol 1991; 15:90-6
- CAO L, YOUNG N, LIU H, et al. Preoperative aspirin use and outcomes in cardiac surgery patients. Ann Surg 2011; 255:399-404
- CARCOANA OV, MATHEW JP, DAVIS E, et al. Mannitol and dopamine in patients undergoing cardiopulmonary bypass: a randomized clinical trial. Anesth Analg 2003; 97:1222-9
- CHENITZ KB, LANE-FALL MB. Decreased urine output and acute kidney injury in the postanesthesia care unit. Anesthesiol Clin 2012; 30:513-26
- CHERTOW GM, BURDICK E, HONOUR M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16:3365-70
- CHIOLERO R, BORGEAT A, FISCHER A. Postoperative arrhythmias and risk factors after open heart surgery. J Thorac Cardiovasc Surg 1991; 39:81-4
- COLEMAN MD, SHAEFI S, SLADEN RN. Preventing acute kidney injury after cardiac surgery. Curr Opin Anesthesiol 2011; 24:70-6
- DEGEUS HRH, RONCO C, HAASE M, et al. The cardiac surgery-associated neutrophil gelatinase-associated lipocalin (CSA-NGAL) score: a potential tool to monitor acute tubular damage. J Thorac Cardiovasc Surg 2016; 151:1478-81
- FUHRMAN DY, KELLUM JA. Epidemiology and pathophysiology of cardiac surgery-associated acute kidney injury. Curr Opin Anesthesiol 2017; 30:60-5
- GAFFNEY AM, SLADEN RN. Acute kidney injury in cardiac surgery. Curr Opin Anesthesiol 2015; 28:50-9
- GARWOOD S. Cardiac surgery-associated acute renal injury: New paradigms and innovative therapies. J Cardiothorac Vasc Anesth 2010; 24:990-1001
- HAASE M, BELLOMO R, HAASE-FIELITZ A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol 2010; 55:2024-33
- HAASE M, HAASE-FIELITZ A, BELLOMO R, et al. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med 2009; 37:39-47
- HABIB RH, ZACHARIAS A, SCHWANN TA, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: Implications on operative outcome. Crit Care Med 2005; 33:1749-56
- HO J, TANGRI N, KOMENDA P, et al. Urinary, plasma, and serum biomarkers' utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis 2015; 66:993-1005
- HORI D, KATZ NM, FINE DM, et al. Defining oliguria during cardiopulmonary bypass and its relationship with cardiac surgery-associated acute kidney injury. Br J Anaesth 2016; 117:733-40
- HOSTE EAJ, VANDENBERGHE W. Epidemiology of cardiac surgery-associated acute kidney injury. Best Pract Res Clin Anesthesiol 2017; 31:299-303
- HU J, CHEN R, LIU S, et al. Global incidence and outcomes of afult patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2016; 30:82-9
- IP-YAM PC, MURPHY S, BAINES M, et al. Renal function and proteinuria after cardiopulmonary bypass: The effects of temperature and mannitol. Anesth Analg 1994; 78:842-847
- JULIER K, DA SILVA R, GARCIA C, et al. Preconditioning by sevoflurane decreases biochemical markers for myocardial and renal dysfunction in coronary artery bypass surgery. Anesthesiology 2003; 98:1315-27
- KARKOUTI K, BEATTIE WC, DATTILO KM, et al. A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery. Transfusion 2006; 46:327-38
- KARKOUTI K, WIJEYSUNDERA DN, YAU TM, et al. Acute kidney injury after cardiac surgery. Focus on modifiable risk factors. Circulation 2009; 119:495-502
- KARKOUTI K, WIJEYSUNDERA DN, YAU TM, et al. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and non-anemic patients. Anesthesiology 2011; 115:523-30
- KELLUM JA, ASPELIN P, BARSOUM RS, et al. for KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2(Suppl):1-138
- LANNEMYR L, BRAGADOTTIR G, KRUMBHOLZ V, et al. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology 2017; 126:205-13
- LASSNIGG A, DONNER E, GRUBHOFER G, et al. Lack of renoprotective effects od dopamine and furosemide during cardiac surgery. J Am Soc Nephrol 2000; 11:97-104
- LEVI A, CROMHEECKE ME, DE JONGE E, et al. Blood loss in cardiac surgery: a meta-analysis of clinical relevant endpoints. Lancet 1999; 354:1940-7
- LIAKOPOULOS OJ, CHOI YH, HALDENWANG PL, et al. Impact of preoperative statin therapy on adverse postoperative outcomes in patients undergoing cardiac surgery: A meta-analysis of over 30,000 patients. Eur Heart J 2008; 29:1548:59
- MACKAY JH, FEERICK AE, WOODSON LE, et al. Increasing organ blood flow during cardiopulmonary bypass in pigs: Comparison of dopamine and perfusion pressure. Crit Care Med 1995; 23:1090-8
- MANGANO DT, TUDOR I, DIETZEL C, et al. The risk associated with aprotinin in cardiac surgery. N Engl J Med 2006; 354:353-65
- MARIK PE. Low-dose dopamine: a systematic review. Intens Care Med 2002; 28:877-83
- MÅRTENSSON J, BELLOMO R. Does fluid management affect the occurrence of acute kidney injury? Curr Opin Anaesthesiol 2017; 30:84-91
- MAZZARELLA V, TACCONE GM, TOZZI C, et al. Renal functions in patients undergoing cardiopulmonary bypass operations. J Thorac Cardiovasc Surg 1992; 104:1625-7
- MEERSCH M, ZARBOCK A. Prevention of cardiac surgery-associated acute kidney injury. Curr Opin Anaesthesiol 2017; 30:76-83
- MEHTA RL, KELLUM JA, SHAH SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31
- MOLNAR AO, PARIKH CR, COCA SG, et al. Association between preoperative statin use and acute kidney injury biomarkers in cardiac surgical procedures. Ann Thorac Surg 2014; 97:2081-7
- NIGWEKAR SU, KANDULA P, HIX JK, et al. Off-pump coronary artery bypass surgery and acute kidney injury: A meta-analysis of randomized and observational studies. Am J Kidney Dis 2009; 54: 413-23
- O'HARA JF. Low-dose "renal" dopamine. Anesthesiol Clin N Am 2000; 18:835-51
- PATEL NN, ROGERS CA, ANGELINI GD, MURPHY GJ. Pharmacological therapies for the prevention of acute kidney injury following cardiac surgery: a systematic review. Heart Fail Rev 2011; 16:553-67
- RANUCCI M, DE BENEDETTI D, BIANCHINI C, et al. Effects of fenoldopam infusion in complex cardiac surgical operations: a prospective, randomised, double-blind, placebo-controlled study. Minerva Anesthesiol 2010; 76:249-59
- RANUCCI M, ROMITTI F, ISGRO G, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg 2005; 80:2213-20
- RETTIG TCD, PEELEN ML, GEUZEBROEK GSC, et al. Impact of intraoperative hypotension during cardiopulmonary bypass on acute kidney injury after coronary artery bypass grafting. J Cardiothorac Vasc Anesth 2017; 31:622-8
- SAGAR UN, KANDULA P. N-acetylcysteine in cardiovascular surgery-associated renal failure: A meta-analysis. Ann Thorac Surg 2009; 87:139-47
- SCHOENWALD PK. Intraoperative management of renal function in the surgical patient at risk. Anesthesiol Clin N Am 2000; 18:719-37
- SETTERGREN G, ÖHQVIST G. Renal dysfunction during cardiac surgery. Curr Opin Anaesthesiol 1994; 7:59-64
- SIMMONS PI, ANDERSON RJ. Increased serum creatinine: A marker for adverse outcome before and after cardiac surgery. Crit care Med 2002; 30:1664-5
- SINCLAIR DG, HASLAM PL, QUINLAN GJ, et al. The effect of cardiopulmonary bypass on intestinal and pulmonary endothelial permeability. Chest 1995; 108:718-24
- SKYTTE LARSSON J, BRAGADOTTIR G, KRUMBHOLZ G, et al. Effects of acute plasma volume expansion on renal perfusion, filtration, and oxygenation after cardiac surgery: a randomized study on crystalloid vs colloid. Br J Anaesth 2015; 115:736-42
- STAFFORD-SMITH M, LI YJ, MATHEW JP, et al. Genome-wide association study of acute kidney injury after coronary bypass graft surgery identifies susceptibility loci. Kidney Int 2015; 88:823-32
- SWAMINATHAN M, STAFFORD-SMITH M. Renal dysfunction after vascular surgery. Curr Opin Anaesthesiol 2003; 16:45-52
- THADHANI R, PASCUAL M, BONVENTRE JV. Acute renal failure. N engl J Med 1996; 334:1448-60
- TOLLER W, HERINGLAKE M, GUARRACINO F, et al. Preoperative and perioperative use of levosimendan in cardiac surgery: European expert opinion. Int J Cardiol 2015; 184:323-36
- TUMLIN JA, FINKEL KW, MURRAY PT, et al. Fenoldopam mesylate in early acute tubular necrosis: a randomized, double-blind, placebo-controlled clinical trial. Am J Kidney Dis 2005; 46:26-34
- URZUA J, TRONCOSCO S, BUGEDO G, et al. Renal function and cardiopulmonary bypass: effect of perfusion pressure. J Cardiothorac Vasc Anaesth 1992; 6:309-12
- YI Q, LI K, JIAN Z, et al. Risk factors for acute kidney injury after cardiovascular surgery: evidence from 2'157 cases and 49'777 controls: a meta-analysis. Cardiorenal Med 2016; 6:237-50
- ZARBOCK A, KELLUM JA, VAN AKEN H, et al. Long-term effects of remote ischemic preconditioning on kidney function in high-risk cardiac surgery patients. Anesthesiology 2017; 126:787-98
- ZHENG Z, JAYARAM R, JIANG L, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med 2016; 374:1744-53