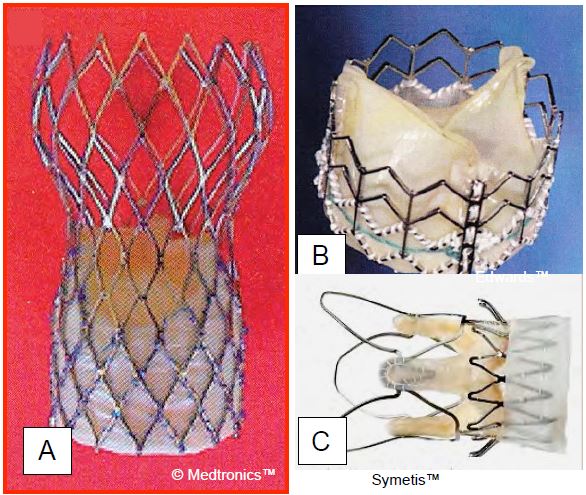
Transcatheter Aortic Valve Implantation (TAVI)
The endovascular implantation of a prosthetic valve without the use of ECC offers the prospect of a significant reduction in mortality in high-risk patients, where it is likely to be >15% with AVR and ECC. This is the reason for TAVI (Transcatheter Aortic Valve Implantation) or TAVR (Transcatheter Aortic Valve Replacement), which can be performed via the femoral route (percutaneous) or the transapical route (left anterior mini-thoracotomy). Many types of bioprosthesis are currently available (three examples are shown in Figure 11.62). These valves are delivered folded over a catheter and deployed when in place at the level of the aortic annulus. The aortic stenosis is first dilated with a balloon. Some valves require balloon dilatation during placement (Sapien™, Sapien XT™), while others are self-expanding and self-deploying (CoreValve™, Symetis Acurate™, JenaValve™). LV ejection is reduced by rapid pacing at 180-220 beats per minute (rapid pacing) when the aortic valve is occluded by the balloon and during prosthesis deployment to avoid embolization. However, self-expanding prostheses do not systematically require rapid pacing. The average gradient of these different prostheses is around 12 mmHg [27].
Video: Deployment of an aortic valve endoprosthesis (TAVI) under rapid electrostimulation (long-axis view).
The primary indication for TAVI is narrow aortic stenosis (S < 0.6 cm2 /m2 , mean gradient > 40 mmHg) in elderly patients (> 70 years) with a life expectancy of > 1 year and an operative mortality estimated by EuroScore of more than 15-20%, or in patients with severe comorbidities with contraindications to ECC: re-operation, thoracic radiotherapy, pulmonary hypertension, right heart failure or porcelain aorta. The current contraindications to TAVI are severe mitral regurgitation, endocarditis, ventricular or atrial thrombus, and dilatation of the ascending aorta (> 4.5 cm) [14]; bicuspid aortic regurgitation and aortic regurgitation are classically considered contraindications, but the development of techniques will undoubtedly allow TAVI to be used in these two situations. In addition, the dimensions of the ring and the aortic root must not exceed those specified for each prosthesis (between 18 mm and 29 mm).
The positioning of the prosthesis is controlled by radiological and echocardiographic visualisation; the use of TEE alone reduces the rate of postoperative renal insufficiency as it avoids the need for contrast injection [12]. It is replaced by the less effective transthoracic exam in cases where sedation and analgesia are used (see Chapter 10, Aortic valve implantation technique).
Video: Image of an aortic valve endoprosthesis in place (long-axis view); the framework of the prosthesis is well supported against the aortic wall; the proximal part is fixed in the subaortic zone of the outflow tract; the movements of the cusps are free.
A comparison between TAVI and AVR in high-risk patients shows that 1-year mortality is lower with TAVI (14.2% vs 19.1%), the complication rate is also lower (20% vs 27%), and the stroke rate is not increased [1]. Compared with surgical AVR, TAVI showed no significant difference in mortality and stroke rates at 2 years (19.3% vs 21.1%), with a reduction in rates of renal failure, AF and bleeding; however, surgical AVR had fewer vascular complications, residual AF and permanent pacemakers [16]. Survival after TAVI is 37% at 5 years (31% at 6 years); one third of patients are in NYHA class I or II; the cumulative stroke rate is 16%. The prosthesis shows no signs of deterioration, with a mean gradient of 10.7 mmHg and a mean surface area of 1.6 cm2 [4]. Despite the advances in prostheses and the remarkable success of the technique, TAVI is limited use for a young, low-risk population and do not yet make it a full equivalent to surgical AVR [18,25].
- The average rate of permanent pacemakers for complete AV block varies between 5% and 25% of cases, depending on the type of prosthesis;
- The rate of significant residual AI (paravalvular and intravalvular leak) remains high (12%) and worsens the prognosis when the insufficiency is grade ≥ 2;
- The rate of stroke is 3-5.5%;
- The long-term durability of prostheses is largely unknown.
Figure 11.62: Three examples of transcatheter aortic valve implantation (TAVI). A: Corevalve™ (Medtronic, Minneapolis, MN, USA); the biological valve is placed inside a self-expanding nitinol stent. B: Sapien™ (Edwards Lifesciences, Irvine, CA, USA); the valve, mounted in a flexible stainless steel stent, is inflated with a balloon during deployment C: Valve Acurate TA™ (Symetis SA, Lausanne, Switzerland), mounted in a self-expanding nitinol stent and used only transapically; it can be repositioned during the procedure, leaving the coronary ostia accessible.
Progress in clinical results is leading to the extension of TAVI to intermediate-risk patients, where the results of AVR in ECC are excellent. At present, there are only a few studies in this category of patients. They tend to show equivalent results with TAVI and AVR in terms of mortality and stroke, but with a higher incidence of residual AI and permanent pacemaker with TAVI, but fewer transfusions, atrial fibrillation and renal failure than surgery [16,30]. The transvalvular gradient is lower with TAVI than with AVR (8 vs 12 mmHg) and the orifice area is larger (2.2 vs 1.8 cm2) [26]. Interim results already show that in moderate-risk patients aged > 65 years operated in experienced centres, the 1-month mortality of TAVI is 1.1-4.4%, which is encouraging and supports the comparison with the mortality of conventional AVR, which is 2.4% > 75 years and 1.3% < 70 years [17]. Long-term follow-up is not available as the longest one for TAVI is 8 years [4].
Percutaneous repair of mitral regurgitation
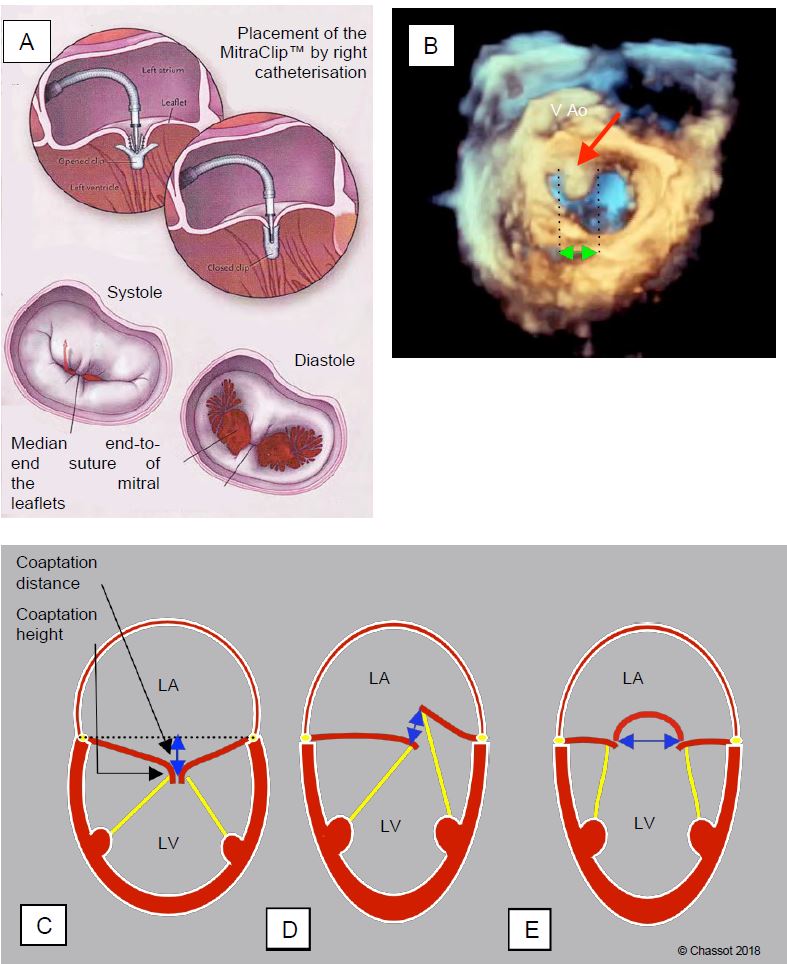
Mitral regurgitation can be treated percutaneously with various types of bioprosthetic and prosthetic devices [28]. Transcatheter mitral valve repair using the MitraClip™ system (Abbott Laboratories) is the most widely used technique. It involves end-to-end suturing of the distal ends of the two mitral leaflets using a clip introduced via the femoral venous route and transeptal access to the LA. The system is based on the Alfieri technique, in which the mitral orifice is transformed into two half-orifices by suturing A2 and P2 together (edge-to-edge repair).
Video: Image of a MitraClip in place in a 70° bi-commissural view.
Video: Three-dimensional view of a MitraClip in place from the LA; the valve opens through two holes on either side of the clip, which joins the central portion of the two leaflets.
Le clip possède deux bras en alliage chrome-cobalt de 8 mm de longueur recouverts de tissu polyester, et deux bras secondaires ou éléments de préhension ; ces derniers permettent d’immobiliser les feuillets au moment de leur accrochage. Le système peut s’ouvrir et se fermer lorsqu’il est monté sur son guide (Figure 11.63) [10,11]. The clip has two 8mm chromium-cobalt alloy arms covered with polyester fabric and two secondary arms or grasping elements; the latter are used to immobilise the leaflets when they are attached. The system can be opened and closed when mounted on its guide (Figure 11.63) [10,11].
Figure 11.63: Percutaneous mitral valve repair using MitraClip™.) A: Suture of the medial end of the two leaflets with a clip; in diastole the orifice is transformed into two hemi-orifices of limited size [11]. A number of conditions must be met for successful mitral clip implantation. B: The width of the prolapse must be < 15 mm (double green arrow) and the prolapse must be located at A2 or P2 (A2 prolapse). C: the height of the coaptation must be > 2 mm and the distance between the coaptation point and the plane of the ring (tenting distance) ≤ 11 mm. D: in the case of prolapse, the gap between the lamellae (interstice) must be < 10 mm. These measurements are made in the mid-esophageal 4-cavity 0-20° and long-axis 120° views of the LV, except for the width of the prolapse, which is more accurate in the full-volume 3D reconstruction. E: The width of the base of the prolapse should not exceed 15 mm in the 60° bicommissural view.
This technique is preferably reserved for situations where the regurgitation is caused by a coaptation defect in the medial region of the leaflets (A2-P2) in symptomatic patients with a very high surgical risk.
- Localised prolapse of P2 or A2;
- Functional MI due to LV dilatation (ventricular failure) or parietal akinesia (ischaemia);
- Elderly or debilitated patients with a predicted mortality rate of more than 15-20% when undergoing PVM in ECC and with a reasonable life expectancy.
To achieve good results, certain conditions must be met; these are well defined for TEE [3,6,23,32].
- Moderate to severe central MI of non-rheumatic or non-endocardial origin;
- Valve orifice area at least 4 cm2, mean gradient ≤ 4 mmHg;
- Coaptation height > 2 mm;
- Distance between the point of coaptation and the plane of the ring ≤ 11 mm (tenting height);
- In case of prolapse, the lesion should be concentrated on a single festoon, preferably A2 or P2; the flail gap should be < 10 mm and the flail width < 15 mm;
- Posterior leaflet length > 10 mm;
- Free edges of flexible, non-calcified leaflets to which the clip is to be applied.
To date, more than 50,000 patients have been treated with MitraClip™. The technical success rate (reduction of MI by ≥ 2 degrees) exceeds 95%, 1-month mortality ranges from 0% to 7.8%, and 1-year survival is between 75% and 90% [20,29]. At 1 year, the incidence of stroke is 1.6% and the reoperation rate is 1.3% [24]. When compared to surgical repair, MitraClip™ has a higher technical failure rate (3.2% vs 0.6%, 25-33% residual MI), but significantly lower operative mortality (3.3% vs 16.2%), stroke rate (1.1% vs 4.5%) and bleeding risk (4.2% vs 59%) in high-risk categories [24,28]. According to international recommendations, the MitraClip™ is indicated for symptomatic patients with severe primary or secondary MI despite optimal medical therapy, who are considered too high risk for surgery (mortality > 8% for MVR and > 6% for MVP) and who have a life expectancy of at least 1 year (class IIb, level of evidence C) [2,22].
Echocardiography plays a crucial role in all percutaneous valve procedures, whether TEE under GA or TEE under sedation, and if involved, the anaesthetist must be familiar with the various plastic or prosthetic devices [13]. For a detailed description of the insertion technique and TEE monitoring, see Chapter 10, MitraClip™ Technique/Imaging and Valvular Interventions.
Anaesthesia for non-invasive valve procedures
The haemodynamic constraints are those of the respective pathologies, essentially aortic stenosis (TAVI) and mitral regurgitation (MitraClip™). They are identical to those encountered in conventional valve surgery, but there are two major differences compared to conventional procedures: 1) no ECC and 2) high-risk patients. For the anaesthetist, these data have two implications for the anaesthetic technique: 1) rapid circuit (extubation on the op table), and 2) rigorous haemodynamic monitoring, invasive monitoring, support as needed. In these procedures, anaesthesia and analgesia need not be deep, as there is no pain stimulation other than that required if resuscitation manoeuvre [15].
For TAVI, the choice of anaesthetic is less important than the way it is administered, which must be very careful: reduced doses, slow induction, immediate correction of any circulatory deviation. Coronary perfusion pressure must be maintained (MAP ≥ 75 mmHg) with a vasopressor (phenylephrine, norepinephrine); this does not mean an increase in LV afterload, as this essentially depends on the degree of aortic stenosis. The greatest haemodynamic stability is achieved with a combination of etomidate/propofol and fentanyl/sufentanil for induction and sevoflurane or propofol for maintenance. The most commonly used technique is total intravenous anaesthesia (TIVA): induction with propofol (2-3 mg/kg) and remifentanil (0.15-0.3 mg/kg), supplemented by rocuronium (0.3 mg/kg) for intubation, followed by continuous infusion of the same agents (propofol 3 mg/kg/hour, remifentanil 0.2 mcg/kg/min) [8,9,15]. Alternatively, induction with etomidate (0.3 mg/kg) or propofol (1-2 mg/kg), sufentanil (0.5-1 mcg/kg) and rocuronium (0.6 mg/kg) followed by maintenance with sevoflurane (1%) and remifentanil (0.2 mcg/kg/min) [7]. In very frail patients, etomidate is preferred to propofol for induction.
While GA is required for thoracic (apical), sternal, axillary or subclavian access, sedation-analgesia is well suited for femoral access. Puncture/arteriotomy is performed under local anaesthetic (infiltration) and/or ilioinguinal or iliohypogastric block. Several methods of sedation/analgesia are available.
- Midazolam: repeated boluses (1-3 mg), possibly combined with nalbuphine (5 mg); risk of delirium (inappropriate > 75 years), prolonged sedation.
- Propofol: infusion (1 mg/kg/h), possibly combined with remifentanil (0.03 mcg/kg/min); reduced preload, risk of respiratory depression.
- Remifentanil: infusion (0.03-0.5 mcg/kg/min) for levels of 1-3 ng/mL; risk of bradycardia and respiratory depression.
- Dexmedetomidine (Dexdor® ): infusion (0.5-1.0 mcg/kg/h); risk of bradycardia and hypotension (hypertension with bolus), contraindicated in AV block.
Sedation-analgesia appears to reduce mortality (RR 0.73), the need for vasopressors (RR 0.45) and length of hospital stay (-2 days), but the incidence of paravalvular leak is higher than with GA (RR 1.2) [31]. There was no difference in the incidence of stroke, renal failure, cardiovascular complications, bleeding or the need for a pacemaker. Anatomically simple cases in co-operating patients in good general condition are performed under LA with the support of transthoracic echocardiography and radiology. GA is reserved for patients in whom implantation may be difficult, who require TEE monitoring due to unfavourable anatomy, or who suffer from renal failure and must avoid contrast product [21]. The trend is certainly towards sedation-analgesia techniques, which are less invasive and more attractive to the patient. Their popularity goes hand in hand with the miniaturisation of the implantation systems, the reduced need for rapid pacing with the new prostheses, the precision of 3D reconstructions and the experience gained in high-volume TAVI centres. Sedation-analgesia is currently used in an average of 60% of cases [5].
After mitral repair, the loss of the low pressure valve, represented by the leak into the LA in systole, is equivalent to an increase in afterload mismatch for the LV as it leaves the bypass graft. In addition, the preload is reduced with the reduction in mitral regurgitation. This situation is particularly worrying in the context of secondary MI, ventricular dilatation and low preoperative EF. This mismatch between afterload and ventricular function is less frequent and less severe with the MitraClip™ technique than with mitral plasty or replacement in ECC, probably because the latter has a deleterious effect on ventricular function [19]. The choice of anaesthetic technique is determined by the patient's condition (extent of MI, ventricular function, comorbidities) and the low invasiveness of the procedure (see Chapter 10, Anaesthesia for MitraClip™).
- Induction: propofol (stable cases) or etomidate (decompensated cases);
- Maintenance: Propofol/remifentanil infusion (stable cases) or isoflurane (decompensated cases); modest doses given the lack of stimulation;
- Curarisation: for intubation only;
- Strict normothermia;
- Heparin 100 IU/kg (ACT 250-300 sec), reversed with protamine;
- Recovery and extubation on the op table or within the first hour.
Valvular stent patients receive lifelong aspirin and dual antiplatelet therapy for 3-6 months.
| Endoprosthetic heart valves |
|
Various non-invasive percutaneous techniques have been developed for valve repair, with the aim of providing a therapeutic option for patients who are too compromised to undergo ECC without the prohibitive operative mortality. Transcatheter aortic valve implantation (TAVI) is virtually equivalent to open surgery (1-year mortality: 25%). The procedure requires one or two brief episodes of low cardiac output during which a transvalvular balloon is deployed to dilate the native valve and expand the prosthesis. To reduce flow during these two periods, rapid ventricular pacing (180-220 beats per minute) is used to temporarily reduce stroke volume and blood pressure (systolic BP ≤ 60 mmHg). Positioning of the prosthesis is monitored by radiological and echocardiographic visualisation. Mitral valve repair for functional regurgitation is essentially achieved by suturing the medial ends of the two leaflets together with a clip (MitraClip™). This technique is intended for high-risk patients with functional MI on LV dilatation; it allows reduction of the MI, not total correction as with surgery, but the operative morbidity and mortality is much lower. Although sedation anaesthesia is possible for femoral approaches, general anaesthesia is required for mini-thoracotomies and for the use of TEE. The choice of anaesthetic is less important than the way it is administered, which must be very careful in these debilitated patients: reduced doses (pain stimulation is low), slow induction, immediate correction of any circulatory deviation. Normothermia must be strictly maintained. The aim is rapid extubation (on the op table). |
References
- ADAMS DH, POPMA JJ, REARDON MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790-8
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BEIGEL R, WUNDERLICH NC, KAR S, SIEGEL RJ. The evolution of percutaneous mitral valve repair therapy. Lessons learned and implications for patient selection. J Am Coll Cardiol 2014; 64:2688-700
- BOULETI C, HIMBERT D, IUNG B, et al. Long-term outcome after transcatheter aortic valve implantation. Heart 2015; 101:936-42
- CERRATO E, NOMBELA-FRANCO L, NAZIF TM, et al. Evaluation of current practices in transcatheter aortic valve replacement. The WRITTEN survey. Int J Cardiol 2017; 228:640-7
- DE BONIS M, AL-ATTAR N, ANTUNES M, et al. Surgical and interventional management of mitral valve regurgitation: a position statement from the European Society of Cardiology working groups on Cardiovascular Surgery and Valvular Heart Disease. Eur Heart J 2016; 37:133-9
- 7ENDER J, BORGER MA, SCHOLZ M, et al. Cardiac surgery fast-track treatment in a postanesthetic care unit. Anesthesiology 2008; 109:61-6
- FASSL J, AUGOUSTIDES JGT. Transcatheter aortic valve implantation - Part 2: Anesthesia management. J Cardiothorac Vasc Anesth 2010; 24:691-9
- FASSL J, WALTHER T, GROESDONK HV, et al. Anaesthesia management for transapical transcatheter aortic valve implantation: A case series. J Cardiothorac Vasc Anesth 2009; 23:286-91
- FELDMAN T, CILINGIROGLU M. Percutaneous leaflet repair and annuloplasty for mitral regurgitation. J Am Coll Cardiol 2011; 57:529-37
- FELDMAN T, FOSTER E, GLOWER DG, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011; 364:1395-406
- FERRARI E, SULZER C, MARCUCCI C, et al. Transapical aortic valve implantation without angiography - Proof of a concept. Ann Thorac Surg 2010; 89:1925-32
- HAHN RT. Transcatheter valve replacement and valve repair. Review of procedures and intraprocedural echocardiographic imaging. Circ Res 2016; 119:341-56
- HOLMES DR, MACK MJ, KAUL S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012; 59:1200-54
- KLEIN AA, WEBB ST, TSUI S, et al. Transcatheter aortic valve insertion: anaesthetic implications of emerging new technology. Br J Anaesth 2009; 103: 792-9
- LEON MB, SMITH CR, MACK M, et al, for the PARTNER 2 investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609-20
- LINKE A, WENAWESER P, GERCKENS U, et al. Treatment of aortic stenosis with a self-expanding transcatheter valve: the international multicentre ADVANCE study. Eur Heart J 2014; 35:2672-84
- MAHMOOD F, MATYAL R, MAHMOOD F, et al. Intraoperative echocardiographic assessment of prosthetic valve: a practical approach. J Cardiothorac Vasc Anesth 2018; 32:823-37
- MELISURGO G, AJELLO S, PAPPALARDO F, et al. Afterload mismatch after MitraClip insertion for functional mitral regurgitation. Am J Cardiol 2014; 113:1844-50
- MUNKHOLM-LARSEN S, WAN B, TIAN DH, et al. A systematic review on the safety and efficacy of percutaneous edge-to-edge mitral valve repair with MitraClip system for high surgical risk candidates. Heart 2014; 100:473-8
- NEUBURGER PJ, PATEL PA. Anaesthetic techniques in transcatheter aortic valve replacement and the evolving role of the anaesthesiologist. J Cardiothorac Vasc Anesth 2017; 31:2175-82
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- O'GARA PT, GRAYBURN PA, BADHWAR V, et al. 2017 ACC Expert consensus decision pathway on the management of mitral regurgitation. J Am Coll Cardiol 2017; 70:2421-49
- PHILIP F, ATHAPPAN G, TUZCU EM, et al. MitraClip for severe symptomatic mitral regurgitation in patients at high surgical risk: a comprehensive systematic review. Cathet Cardiovasc Interv 2014; 84:581-90
- PURI R, CHAMANDI C, RODRIGUEZ-GABELLA T, et al. Future of transcatheter aortic valve implantation - evolving clinical indications. Nat Rev Cardiol 2018; 15:5765
- REARDON MJ, VAN MIEGHEM NM, POPMA JJ, et al, for the SURTAVI Invesitgators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017; 376:1321-31
- SELLEVOLD OFM, GUARRACINO F. Transcutaneous aortic valve implantation: recent advances and future. Curr Opin Anaesthesiol 2010; 23:67-73
- SORAJJA P, LEON MB, ADAMS DH, et al. Transcatheter therapy for mitral regurgitation. Clinical challenges and potential solutions. Circulation 2017; 136:404-17
- TARAMASSO M, GAEMPERLI O, MAISANO F. Treatment of degenerative mitral regurgitation in elderly patients. Nature Rev Cardiol 2015; 12:177-83
- THYREGOD HG, STEINBRUCHEL DA, IHLEMANN N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol 2015; 65:2184-94
- VILLABLANCA P, MOHANANEY D, NIKOLIC K, et al. Comparison of general versus local anesthesia in patients undergoing transcatheter aortic valve replacement (TAVR): a meta-analysis. Catheter Cardiovasc Interv 2018; 91:330-42
- ZAMORANO JL, BADANO LP, BRUCE C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur J Echocardiogr 2011; 12:557-84