In this category, non-cyanotic heart diseases such as ASD, VSD, AV canal defects, partial anomalous pulmonary venous return and patent ductus arteriosus overload the pulmonary circulation (see Figures 14.8 and 14.14). The chamber downstream of the shunt is dilated: a shunt upstream of the atrioventricular valves creates a volume overload for the RV while a shunt downstream of these valves prompts a volume overload for the LV (see Figure 14.11). In both cases, LA pressure is high. To ensure circulation of the additional volume through the shunt, cardiac output must rise through tachycardia and increased stroke volume. Leakage of arterial blood into the low-pressure pulmonary circuit reduces the systemic diastolic pressure. This is particularly pronounced in subjects with a large ductus arteriosus. The high cardiac output (high mVO2) and low diastolic pressure incur a risk of myocardial ischaemia even if the coronary arteries are normal.
L-to-R shunts may result in pulmonary arterial hypertension (PAH). This is particularly severe and fast if the pressure through the shunt is high. PAH causes pressure overload in the RV, which hypertrophies (RVH). The shunt blood flow (Qp/Qs ratio) varies depending on the PVR to SVR ratio. It falls if SVR drops (reduced upstream pressure) and if PVR rises (increased downstream pressure) (see Figures 14.8). The shunt can therefore be reduced through systemic arterial vasodilation and pulmonary vasoconstriction by hypoventilation. If the diameter of the L-to-R defect exceeds 75% of aorta diameter, the aortic and pulmonary systolic pressure equalises. The shunt blood flow becomes strictly proportional to vascular resistance. It may even be reversed if PVR rises and SVR falls [1].
Although these heart diseases show very few symptoms at birth, infants’ clinical condition deteriorates at the end of the first month of life when PVR falls significantly. The L-to-R shunt increases and ventricular failure becomes evident – tachycardia, pulmonary congestion, fatigue and dyspnoea during feeding, growth retardation.
The situation for palliative shunts (e.g. Blalock-Taussig) and aortopulmonary collaterals is very different since the pulmonary blood flow is reduced. The shunt blood flow, which supplies the pulmonary circulation, is proportional to systemic pressure. A drop in SVR causes it to fall, with hypotension triggering a reduction in SpO2 (see Figure 14.10). The systemic diastolic pressure is low due to the loss of blood volume into the low-pressure pulmonary circuit.
Implications for anaesthesia
L-to-R shunts may result in pulmonary arterial hypertension (PAH). This is particularly severe and fast if the pressure through the shunt is high. PAH causes pressure overload in the RV, which hypertrophies (RVH). The shunt blood flow (Qp/Qs ratio) varies depending on the PVR to SVR ratio. It falls if SVR drops (reduced upstream pressure) and if PVR rises (increased downstream pressure) (see Figures 14.8). The shunt can therefore be reduced through systemic arterial vasodilation and pulmonary vasoconstriction by hypoventilation. If the diameter of the L-to-R defect exceeds 75% of aorta diameter, the aortic and pulmonary systolic pressure equalises. The shunt blood flow becomes strictly proportional to vascular resistance. It may even be reversed if PVR rises and SVR falls [1].
Although these heart diseases show very few symptoms at birth, infants’ clinical condition deteriorates at the end of the first month of life when PVR falls significantly. The L-to-R shunt increases and ventricular failure becomes evident – tachycardia, pulmonary congestion, fatigue and dyspnoea during feeding, growth retardation.
The situation for palliative shunts (e.g. Blalock-Taussig) and aortopulmonary collaterals is very different since the pulmonary blood flow is reduced. The shunt blood flow, which supplies the pulmonary circulation, is proportional to systemic pressure. A drop in SVR causes it to fall, with hypotension triggering a reduction in SpO2 (see Figure 14.10). The systemic diastolic pressure is low due to the loss of blood volume into the low-pressure pulmonary circuit.
Implications for anaesthesia
In pharmacokinetic terms, intravenously injected substances are diluted by arterialised blood added by the shunt. These They reach the arteries later and their circulating concentration is prolonged (extended half-life) (Figure 14.18)
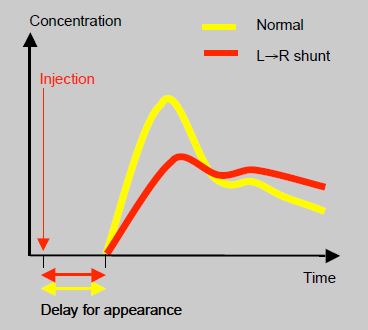

Figure 14.18: Impact of a L→R shunt on the the delay between the intravenous injection of a substance and its blood concentration in the arterial circulation. Yellow: normal. Red: patient with a L→R shunt.
Induction with intravenous agents is slowed. In contrast, uptake and elimination of inhaled agents is accelerated due to the high pulmonary blood flow – blood returning from the lungs goes back through the pulmonary artery, increasing its dissolved gas content and therefore blood concentration is higher [3]. In practice, this effect is only clinically detectable if the shunt is higher than 60% and the systemic blood flow is low, thus increasing the proportion of the cardiac output perfusing the brain [6]. From a respiratory perspective, pulmonary vascular overload reduces lung compliance and increases work of breathing [7].
The twofold objective of increasing PVR and lowering SVR determines which anaesthetic techniques should ideally be used and how haemodynamics should be managed:
The twofold objective of increasing PVR and lowering SVR determines which anaesthetic techniques should ideally be used and how haemodynamics should be managed:
- To lower SVR: general anaesthesia with isoflurane or sevoflurane, possibly an arterial vasodilator (phentolamine, sodium nitroprusside). In noncardiac surgery: spinal or epidural anesthesia.
- Relative hypoventilation is induced to increase PVR:
- FiO2: 0.21 - 0.3;
- Hypoventilation with moderate hypercapnia (PaCO2 45 mmHg);
- Hyperbaric ventilation with PEEP (increasing RV afterload);
- By adding CO2 to the inhaled gases (FiCO2: 2-4%) it is theoretically possible to reduce diversion of blood from the systemic circuit to the low-pressure pulmonary circuit without having to hypoventilate the patient with an excessively low tidal volume [2]. This step should also increase SVR, although it is not widely practised. Some centres use it in the event of circulatory arrest in ECC under CPB [5].
- Maintaining relative hypervolaemia – hypovolaemia exacerbates the shunt due to the inevitable sequestration of volume in the low-pressure pulmonary circuit. CVP is not a good indicator of filling preload.
- By increasing Ht, it is possible to raise PVR and slow the L-to-R shunt [4].
Once the shunt is closed, the patient is hypervolaemic since the volume associated with the shunt no longer circulates in the pulmonary circuit, but overloads the systemic circulation.
| Left-to-right shunt ( Qp ↑ ) |
|
ASD, VSD, AV canal defect, partial anomalous pulmonary venous return, patent ductus arteriosus, L-to-R fistula
To reduce the shunt: - ↓ SVR (arterial vasodilator, isoflurane, spinal anaesthetic, epidural neuraxial blockade) - ↑ PVR (hypoventilation, FiO2 0.21-0.3, PEEP, add CO2 (2-4%) - Prevent hypovolaemia and anaemia |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- CASSORLA L. Preoperative evaluation and preparation : A physiologic approach. In : ANDROPOULOS DA, et al, eds. Anesthesia for congenital heart disease. Oxford: Blackwell-Futura, 2005, 175-96
- JOBES DR, NICOLSON SC, STEVEN JM, et al. Carbon dioxide prevents pulmonary overcirculation in hypoplastic left heart syndrome. Ann Thorac Surg 1992; 54:150-1
- LAIRD TH, STAYER SA, RIVENES SM, et al. Pulmonary-to-systemic blood flow ratio effects of sevoflurane, isoflurane, halothane and fentanyl/midazolam with 100% oxygen in children with congenital heart disease. Anesth Analg 2002; 95:1200-6
- McGRATH RL, WELL JV. Adverse effects of normovolemic polycythemia and hypoxia on hemodynamics in the dog. Circ Res 1978; 43:793-8
- TABBUTT S, RAMAMOORTHY C, MONTENEGRO LM, et al. Impact of inspired gaz mixtures on preoperative infants with hypoplastic left heart syndrome during controlled ventilation. Circulation 2001 ; 104 :1159-64
- TAKKENBERG JJM, KLIEVERIK LMA, SCHOOF PH, et al. The Ross procedure: a systematic review and meta-analysis. Circulation 2009; 119:222-8
- THORSTEINSSON A, JONMARKER C, LARSSON A, et al. Functional residual capacity in anesthetized children: normal values and values in children with cardiac anomalies. Anesthesiology 1990; 73:876-81
