- No long-term anticoagulation (anticoagulation for 3 months with an INR target of 2.0-2.5 during endothelialisation of the prosthetic ring, followed by lifelong aspirin);
- Preservation of ventricular function (ventricular geometry and integrity of the subvalvular apparatus are preserved after mitral or tricuspid valve repair);
- Very low risk of thromboembolic and infectious (endocarditis) complications;
- Virtually no risk of structural deterioration;
- Possible valve growth in children and adolescents;
- Mortality 2-4 times lower.
The low mortality (average 1%) and long-term success (10-year revision rate: 6-14%) are an incentive to intervene earlier in the course of the disease [6]. Further details of the surgical procedure can be found in the chapters on the individual valves. The technique for mitral valve plasty (MVP) is adapted to the type of lesion (Figures 11.50 and 11.51). Of the many possible techniques, the following are the most commonly used [19].
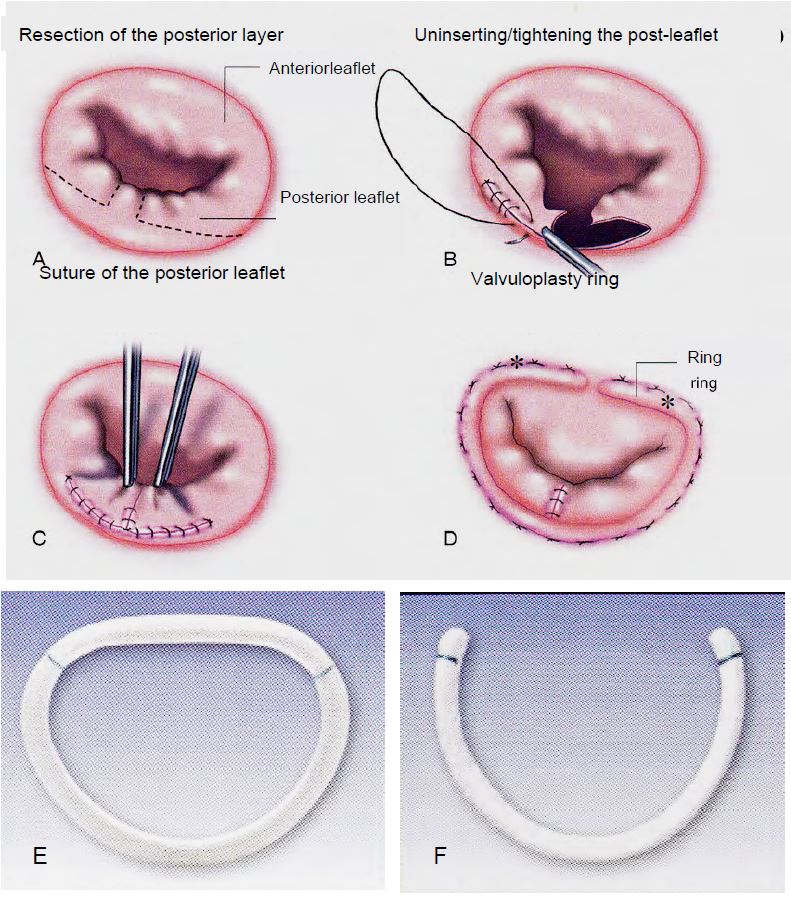
Figure 11.50: Mitral plasty for P2 prolapse and prosthetic rings. A: Square resection of P2. B: Desinsertion, sliding and suturing of the posterior leaflet at the level of P1 and P3. C: Suture of the posterior leaflet. D: Placement of a classic Carpentier-Edwards valvuloplasty ring. The 2 asterisks (*) mark the intertrigonal distance corresponding to the ring (Doty DB). Cardiac Surgery: Surgical Technique. St Louis: Mosby Year Book, 1997). E: Carpentier-Edwards physio ring. F: Cosgrove-Edwards ring [3].
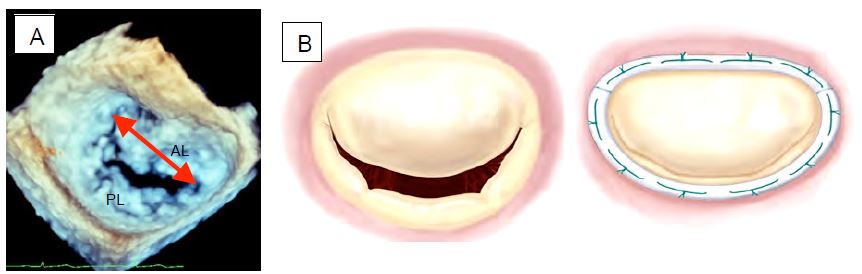
- Annulopasty with a flexible or rigid ring (Carpentier-Edwards™, Duran™, ETlogics™, GeoForm™). The size of the ring depends on the intertrigonal distance we want to achieve after the procedure (intertrigonal distance = 0.8 - length of the anterior leaflet base in 60° bicommissural view with clockwise rotation of the probe or in 3D en face view) (Figure 11.52A). The size varies from 28 to 36 mm in structural MI and from 26 to 32 mm in functional MI. This ring is essential to prevent secondary dilatation and ensure long-term success. It has four functions: to restore the geometry of the mitral annulus, to prevent dilatation, to reduce tension on the sutures and to increase the coaptation area (Figure 11.52B).
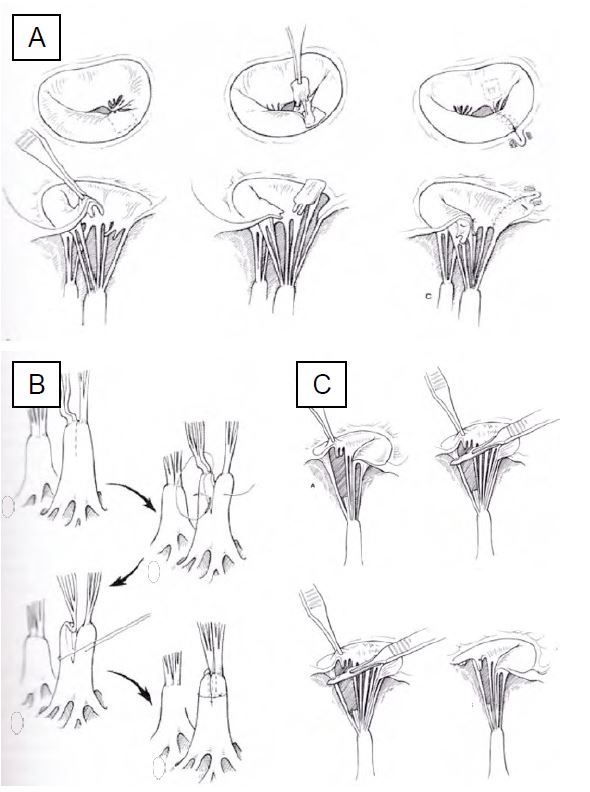
Figure 11.51: Mitral repair. A: Transposition of the cords for prolapse of the anterior leaflet. B: Cord shortening technique. C: Repair of a torn chord with translation of a secondary chord to the free edge [Edmunds LH. Cardiac surgery in the adult. New-York :McGraw-Hill, 1997].
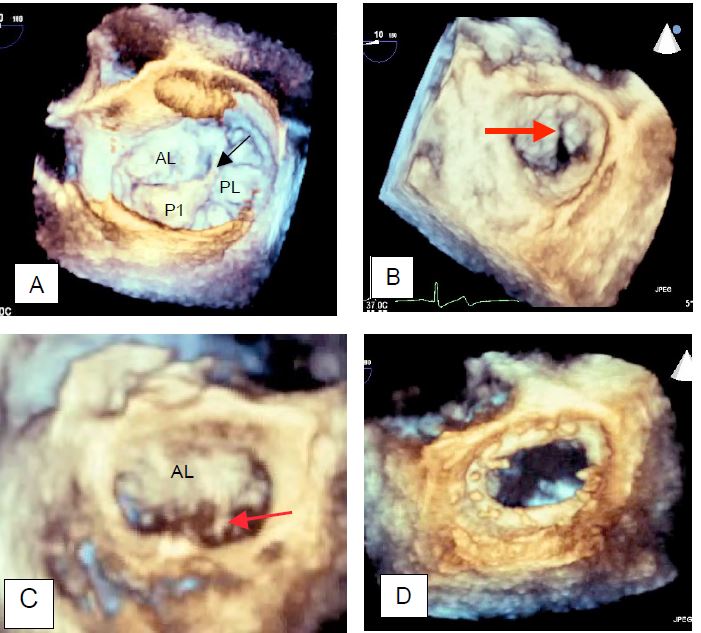
Figure 11.52: Mitral valve annuloplasty. A: 3D TEE reconstruction of the mitral valve "en face" seen from the LA. Measurement of the intercommissural distance. AL: Anterior leaflet. PL: Posterior leaflet. B: Restrictive effect of the prosthesis ring in coaptation of the leaflets.
- Quadrangular resection of the leaflet(s) of the posterior leaflet (P2 in 57% of cases) and resection of the torn chords; suturing of the posterior leaflet, possible re-insertion and sliding valvuloplasty of P1 and P3 to reduce the leaflet height to <1.5 cm and reduce the risk of SAM in the case of extensive resection and a very large posterior leaflet (see HOCM effect after MVP below).
Video: Echocardiographic view of the mitral valve after plasty-resection of the posterior leaflet; only the anterior leaflet closes the valve. The plasty is correct because the anterior leaflet abuts under the posterior annulus during systole, ensuring a seal.
- Triangular resection of the anterior leaflet, resection of broken cords, edge-to-edge suture; transfer of secondary cords to the free edge. In case of extensive resection: transfer of a festoon of the posterior leaflet and its chords. The results of anterior leaflet plasty, which is more difficult, are not as good as those of posterior leaflet plasty.
Video: Aspect of the mitral valve after plasty in a case of congenital malformation; the anterior leaflet is reshaped but the two leaflets are pressed against each other in systole.
- Transfer, shortening, reinsertion or replacement of chords (Gore-Tex™ Chord); chords are attached to the leaflet on patches and buried in the papillary muscle; the exact length of neochords is often difficult to assess.
- Decalcification of the mitral annulus; in long-term prolapse or calcific disease, calcium is deposited in the reactive fibrosis of the posterior part of the annulus. The posterior leaflet is removed, the annulus is decalcified en bloc and the atrio-ventricular junction is reconstructed by repositioning the atrial wall on the ventricular musculature and, if necessary, adding a pericardial patch.
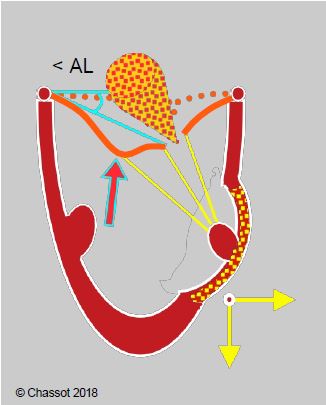
- In secondary ischaemic MI with external displacement of the cusps and excessive traction on the central part of the anterior leaflet: resection of the 2nd order chords of the anterior leaflet (Figure 11.65B) [21].
Figure 11.65B: Segmental akinesia or dyskinesia driving the papillary muscle outwards with excessive traction on the 2nd order chords of the anterior leaflet in ischaemic mitral regurgitation, resulting in a gull-wing appearance of the anterior leaflet. < AL: angle of the anterior leaflet at its tip with the plane of the annulus in systole. Surgical division of the 2nd order chords can be curative in this case.
- Alfieri plasty: edge-to-edge suture of the medial part of the leaflets (A2 and P2), reserved for very high-risk cases with functional MI and central leak, as well as refractory SAM after MVP; in diastole, the valve presents 2 hemi-orifices of reduced dimensions. This technique is performed transcutaneously (MitraClip™) (see Valvular Endoprostheses).
Video: Echocardiographic appearance after MitraClip™ placement in a 70° bi-commissural view.
Video: View of two simultaneous perpendicular planes ("X-plane") after MitraClip insertion; the colour flow shows only a minor leak in systole; the diastolic flow through the valve is unobstructed.
- Closure of an opening (endocarditis) or cleft (cleft) by a patch of pericardium.
- Partial replacement of the leaflets or lengthening of the anterior leaflet with autologous pericardium or a homograft.
Echocardiography
A pre ECC echocardiogram (TEE) provides a dynamic view of the valve and allows us to assess whether surgery can be performed under satisfactory conditions. In particular, we look for
- Quantification of MI (tendency to underestimate in GA): PISA, vena contracta;
- Mechanism of MI: constriction, prolapse, cord rupture, dilatation;
- Exact location of origin of MI jet(s);
- Leaflet mobility, length and thickness; location of abnormalities;
- Condition of 2 commissures;
- Condition of annulus (calcifications) and subvalvular apparatus;
- Mitral and pulmonary venous flow;
- LV function and size; LA size;
- Specific measurements (see below): annular diameter, intercommissural distance, anterior leaflet length.
- In restrictive secondary MI; tentorial distance, leaflet angle in systole.
Certain echocardiographic criteria can be used to predict good feasibility and outcome of the procedure [2,15,18,24].
- Ring diameter < 5.0 cm (measured in mid-esophageal bicommissural 40-60° and long-axis 120-140° views);
- Anterior leaflet length to annular diameter ratio > 0.65 (measured in diastole and 120-140° long axis view);
- Lesion of 1 or 2 posterior leaflets, stable commissures;
- Degree of moderate to severe MI;
- Single jet;
- Satisfactory subvalvular apparatus;
- No calcification of the annulus.
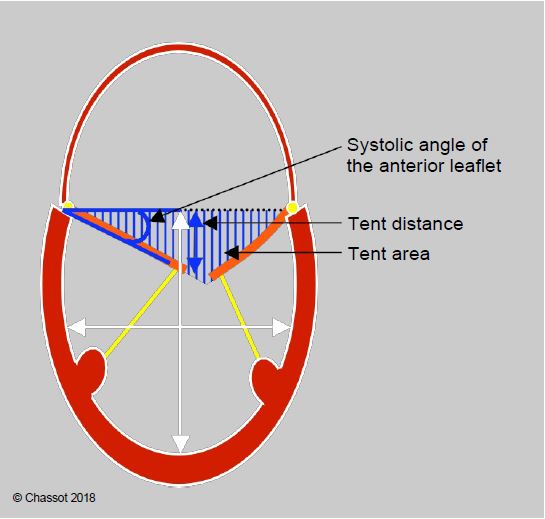
For restrictive secondary MI (IIIb), the criteria are slightly different (Figure 11.53):
Figure 11.53: Restrictive MI (type IIIb) in a dilated LV. The degree of restriction is defined by the distance from the point of coaptation to the plane of the mitral annulus (tenting distance), the triangular area between the leaflets and the plane of the annulus (tenting area), and the angle of closure of the anterior leaflet measured between the plane of the annulus and the tip of the leaflet [22]. The ratio of LV long to short axis diameter defines the degree of sphericity (normal long to short axis ratio: > 1.5).
- Distance ring plane - coaptation point (tenting height) < 1.0 cm;
- Tenting area < 1.5 cm2 ;
- Anterior leaflet angle of closure < 25°;
- Posterior leaflet closure angle < 45°;
- LV sphericity index ≥ 1.5 (no significant dilatation).
Three-dimensional imaging provides a more accurate picture of the anatomy and motion of the mitral valve. In particular, it can be used to determine the size of the mitral annulus to be implanted based on measurements of the intercommissural distance, anterior leaflet height and annulus diameters (Figure 11.54) [7,11,13,23].
Video: Prolapse of the anterior part of the posterior leaflet (P1) in 3D reconstruction viewed from the LA.
Video: Prolapse of the medial part of the posterior leaflet (P2) in 3D reconstruction viewed from the LA.
Figure 11.54: Examples of three-dimensional imaging of the mitral valve. A: Mitral valve with prolapse of the anterior festoon of the posterior leaflet (P1); the arrow indicates a tear in the chord. B: Prolapse of the anterior leaflet; this condition can be very difficult to see in 2D, but is clearly visible in 3D. C: Mitral valve with prolapse of the posterior part of the anterior leaflet (A3); the arrow indicates a torn chord. D: Image of a mitral prosthesis ring seen from the LA.
Some data suggest that success is unlikely and recurrence is common (>50% of cases) [5,12,14,16,18].
- Involvement of ≥ 3 festoons;
- Involvement of 2 leaflets;
- Connected, poorly developed leaflets, commissural fusion (ARF; endocarditis);
- Length of anterior leaflet < 2.8 cm and posterior leaflet < 1.7 cm;
- Very large regurgitant orifice (> 0.4 cm2);
- Massive annular dilatation (diameter > 5.0 cm in structural MI and > 4.0 cm in functional MI) or very restrictive annulus (diameter < 3.5 cm);
- Extensive calcification.
In secondary ischaemic MI, annular diameter > 37 mm, tent height > 11 mm, tent area > 2.0 cm2 on TEE and > 1.6 cm2 on intraoperative TEE, anterior leaflet angle > 25°, posterior leaflet angle > 45° and severe colour jet MI are indicators of a 50% risk of recurrence [12]. Severe LV dilatation (telesystolic diameter > 6.0 cm, sphericity index > 0.7, telesystolic papillary muscle width > 2.5 cm) is also a handicap [18].
TEE examination after ECC
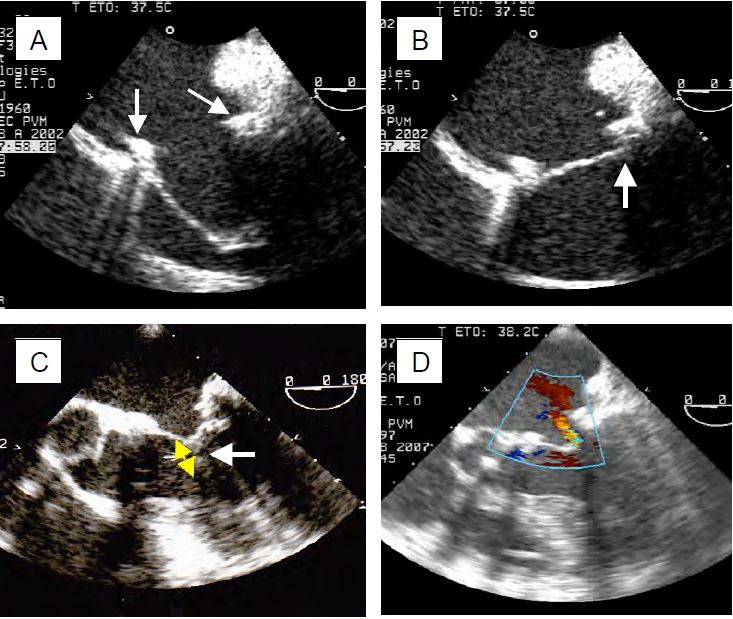
The TEE image of the valve post-procedure may appear bizarre, as restoration of normal function is not synonymous with normal anatomy. In general, the posterior leaflet acts as a stop for the anterior leaflet, which opens and closes by itself (unicuspid valve). After ECC, the echocardiographic criteria for successful mitral valve repair are defined as normal afterload (blood pressure), preload (blood volume) and ventricular function. The examination must be accompanied by an afterload test (MAP > 80 mmHg, PAsyst > 120 mmHg) and contractility stimulation to ensure that the results obtained correspond to real-life situations. The procedure is considered successful if the following conditions are met (Figure 11.55) [9,19].
Figure 11.55: TEE images after mitral valve repair. A: After wide resection of P2 and plasty of the posterior leaflet, complete occlusion is ensured by the anterior leaflet; its length must exceed the diameter of the annulus (arrows) and allow contact under the posterior leaflet for the valve to be occluded. B: Satisfactory occlusion of the anterior leaflet under the posterior leaflet and the annulus in systole. C: Coaptation of the leaflets must be at least 4 mm high (yellow arrow) to ensure a seal, which is the case here after plasty and resection of the anterior leaflet. D: Residual leak after mitral plasty; if it is pansystolic, the leak is excessive because the jet is significantly wide as it passes through the valve.
- Trivial or small residual MI (vena contracta < 3 mm, regurgitant orifice < 0.2 cm2 ); protosystolic and brief MI.
Video: Colour flow image after plastic resection of the posterior mitral leaflet; the anterior leaflet abuts under the posterior leaflet without leakage in systole.
- If the posterior leaflet is resected: the anterior leaflet is placed under the posterior leaflet and the posterior part of the ring.
Video: Image after plastic resection of the posterior mitral leaflet; the anterior leaflet rests on the posterior leaflet to a limited extent.
- If the 2 leaflets meet: coaptation author > 5 mm; this height is calculated by subtracting the distance between the mitral annulus and the coaptation point in systole from the total length of the anterior leaflet measured in diastole (120° long axis view) (see Figure 11.22).
- Ratio of coaptation height to anterior leaflet length > 0.2.
- Ratio of posterior leaflet length to anterior leaflet length < 0.4.
- Anterograde gradient: ΔPmax ≤ 4 mmHg, ΔPmoy ≤ 2 mmHg.
- Vmax ≤ 1.5 m/s in the LVOT, no SAM (see below "HOCM effect" after MVP).
Echocardiography may indicate the need to return to the pump to complete the procedure or, failing that, to replace the valve [8].
- Structural defect that cannot be improved: perforation, dehiscence, leaflet tilt, para-annular leak;
- MI of more than mild degree:
- Non-adaptation of 2D or 3D images;
- Large, pansystolic MI jet;
Video: Major residual mitral regurgitation after mitral plasty; correction is essential.
- Presence of a PISA on the ventricular side;
- Vena contracta > 0.3 cm;
- Regurgitant orifice > 0.25 cm2;
- Regurgitant volume > 25 mL;
- Restrictive stenosis: S < 2 cm2 , ΔPmax ≥ 10 mmHg, ΔPmoy ≥ 5 mmHg; gradient tends to decrease by 20-30% during the first postoperative year;
- SAM refractory to medical treatment (see below);
- The decision is based on the clinical context and not on the TEE image alone.
Measuring the gradient, the useful area in diastole and the area of the regurgitant orifice in systole is complex because the orifice area is not circular. In addition, the axis of flow is oblique to the Doppler beam because of the inclined funnel shape created by plasty. Calculation using the pressure half-time is unsuitable for the irregular geometry of the orifice and the gap between the axis of diastolic flow and the Doppler axis. Under these conditions, planimetry is more accurate after careful cropping of the 3D reconstruction. An excessive gradient may occur in the presence of fluid overload (too rapid transfer of ECC volume) or high cardiac output (excess catecholamines). These factors must be checked before deciding to return to ECC. Conversely, low cardiac output, severe diastolic dysfunction or associated aortic insufficiency may mask the reality by artificially reducing the diastolic gradient [8].
It is important to diagnose the mechanism of the residual insufficiency so that the surgeon can make an appropriate correction [9].
- Type I MI (central jet in the LA): ring too large, iatrogenic perforation, residual gap;
- Type II MI (eccentric jet directed away from the prolapsing leaflet): residual prolapse, the location of which remains to be precisely defined;
- Type III MI (eccentric jet towards the prolapsing leaflet): excessive resection, malposition of the annulus, excessive shortening of the cords, ventricular dilatation;
- MI due to SAM: mesosystolic jet, posterior leaflet too long, excess valve tissue, annulus too small.
When applying a valvuloplasty ring, it is important to check three things after weaning off pump.
- Lateral wall contractility; the circumflex artery (CX) may be injured by the lateral fixation points.
- Sealing of the aortic valve; sutures at the base of the anterior leaflet may exert traction on the left cusp or non-coronary cusp of the aortic valve and cause an AI; they may also perforate an aortic cusp.
- Integrity of the basal wall of the LV; decalcification of the annulus can lead to lesions in the atrioventricular groove; persistent presence of air in the LV and basal akinesia are warning signs of ventricular rupture.
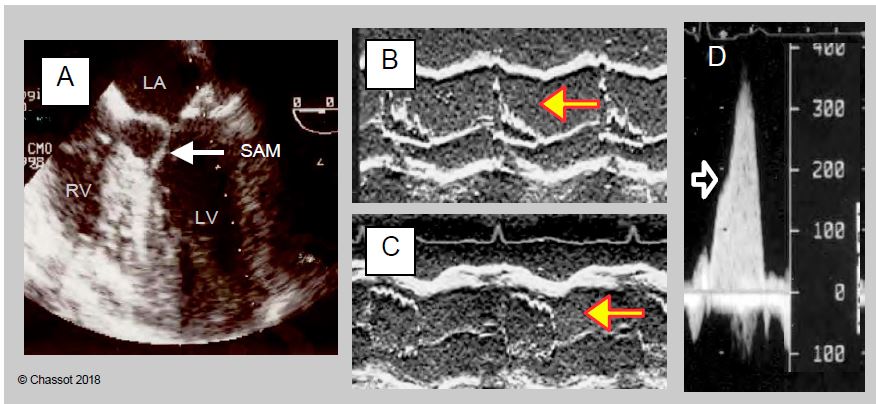
"HOCM effect" after MVP
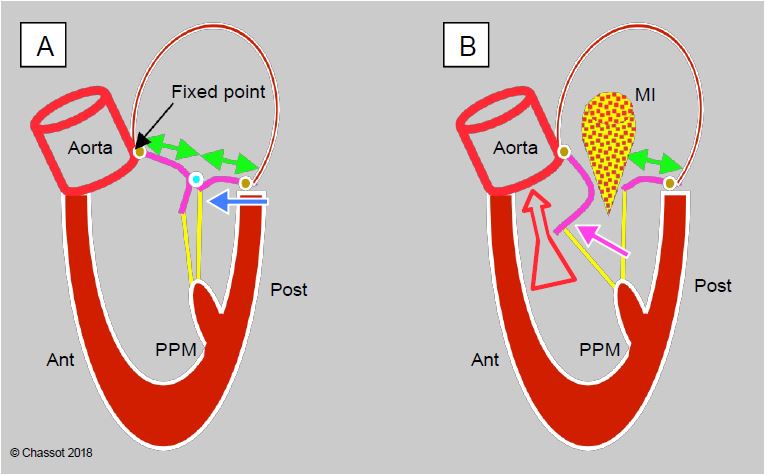
Dynamic subobstruction of the outflow tract by the anterior mitral leaflet (obstructive cardiomyopathy effect or HOCM effect) occurs in 4-12% of mitral valve replacements for myxoid degeneration (MI type II) (Figure 11.56) [10,20]. It is secondary to the anterior displacement of the mitral coaptation zone by a restrictive ring in a patient with an abundance of valve tissue; this displacement brings it excessively close to the LVOT. In fact, the fixed point of the mitral annulus is at the mitoaortic junction (fibrous trine), whereas the posterior part is thin and supported only by the ventricular musculature; it is this part that will move anteriorly. This displacement occurs for four different reasons.
Figure 11.56: Dynamic obstruction of the LVOT after mitral valve repair. A: A restrictive annulus and/or excessive posterior leaflet (PL) height (anterior/posterior leaflet ratio < 1.3, green arrows) advances the posterior wall and projects the coaptation point towards the LVOT (blue arrow). In the protocole, coaptation takes place on the body of the anterior leaflet (AL), the distal part of which is in the LV and not against the PL. B: During mesosystole, intraventricular pressure pushes the AL towards the LVOT (purple arrow); during ejection, it is sucked in by the Venturi effect and blocks the LVOT (SAM: systolic anterior motion). The aortic flow suddenly drops and the reopening of the mitral valve causes meso-tesystolic insufficiency.
- The valvuloplasty ring is too restrictive.
- The length of the posterior leaflet is excessive (excess tissue typical of Barlow's disease).
- The ventricular cavity is too small: hypovolemia, concentric LVH.
- The radial displacement of the posterior wall during systole is too great: over-stimulation β , vasoplegia.
The first two are related to the surgical procedure and occur in 5% of mitral valve repair cases [10]. The other two are related to haemodynamics and are corrected by filling, systemic vasoconstriction and β-blockage; they are much more common but do not require reoperation.
When the coaptation point of the mitral valve moves towards the LVOT, the occlusion is no longer on the distal edge of the anterior leaflet but on its body, and the intaventricular pressure pushes it anteriorly towards the outflow tract in protosystole. Due to the Venturi effect, it is then pulled into the LVOT and occludes it more or less completely. This is the SAM (systolic anterior motion) that occurs in meso-tesystole (Figure 11.57). The flow in the aorta decreases and the reopening of the mitral valve during systole causes a meso-tesystolic MI. This phenomenon is clearly visible in the 4-cavity, 5-cavity view or long-axis mid-esophageal LV.
Video: Image of displacement of the anterior mitral leaflet (AML) in the outflow tract after mitral plasty
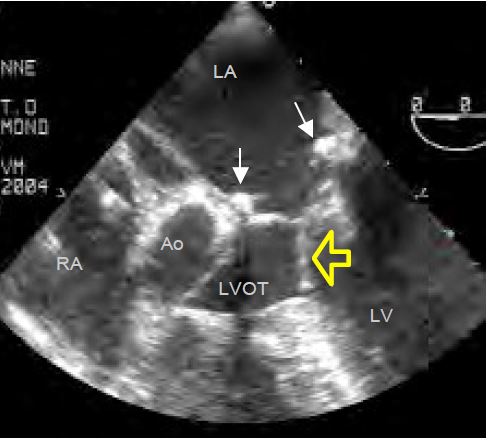
- Distance between the mitral coaptation point and the septum (C-sept) < 2.6 cm.
- Posterior leaflet height > 1.5 cm (measured in diastole in the plane of its greatest dimension).
- Ratio of anterior leaflet height to posterior leaflet height ≤ 1.3.
- Distance between the coaptation point and the plane of the mitral annulus > 0.6 cm.
- Closed mitral valve angle (< 140°).
- Significant MI but not holosystolic;
- Inflection of the anterior leaflet in the chase chamber during systole (SAM).
- Vmax in the LVOT > 2.5 m/s (in 0° deep transgastric view or 120° long axis of the LV);
- Dagger-shaped appearance of aortic flow on continuous Doppler;
- Mesosystemic collapse of the aortic cusps in TM mode of the aortic valve (short axis 40° or long axis 120°);
- Meso-tele-systolic MI.
- Therapeutic test: if correction of haemodynamics eliminates the HOCM effect, redo surgery is not necessary;
- Optimise the preload (filling);
- Increase afterload (repeated bolus of 100 mcg phenylephrine, infusion of norepinephrine);
- Decrease contractility (discontinuation of catecholamines β, repeated bolus of 10 mg esmolol).
- Provocative test: used to determine whether the deterioration in haemodynamic conditions is tolerable (maintenance of cardiac output) or whether it leads immediately to cardiogenic shock;
- Decrease in preload: infusion of nitroglycerin;
- Increase contractility: infusion of dobutamine (5-10 mcg/kg/min).
- Resistance to the provocative test provides a good haemodynamic safety margin for the postoperative period. However, the test should be performed before decannulation to maintain a functional ECC in the event of circulatory collapse. It is only indicated in doubtful cases.
- Disappearance of SAM;
- Reduction of Vmax in the LVOT to ≤ 1.5 m/s;
- Disappearance or reduction of MI to a trace (degree < minor);
- Disappearance of mesosystolic collapse of the aortic cusps (the most robust criterion).
| Mitral valve plasty (MVP) |
| Valvuloplasty, which can be performed in 60-80% of cases of mitral regurgitation, is always preferable to replacement because it restores normal anatomy. Advantages over prosthesis
- No anticoagulation
- Preservation of ventricular geometry and function
- Very low mechanical and infectious risks
- Three times lower operative mortality (average 1%); 10-year revision: 6-10%.
MVP consists of resection of the prolapsing leaflet (usually P2), resection of torn leaflets, shortening or transfer of leaflets and implantation of a ring (necessary to prevent secondary dilatation).
Feasibility criteria for mitral valve repair:
- Moderate dilatation of the LV and LA (mitral annulus diameter < 5 cm)
- Low leaflet obstruction (distance from ring plane to coaptation point < 1 cm)
- Flexible, non-calcified ring
- Non-massive MI, stable commissures, single jet
- Anterior leaflet length to ring diameter ratio > 0.65
- Satisfactory subvalvular apparatus
Criteria for successful mitral valve repair (post-ECC study, average failure rate 6%) :
- No or minimal residual MI (MAP > 80 mmHg, satisfactory LV function)
- Adequate leaflet contact (coaptation height > 5 mm), no prolapse
- Mean anterograde gradient ≤ 2-3 mmHg
- No SAM, Vmax LVOT < 1.5 m/s
Dynamic post-procedural subaortic stenosis (4-12% of cases):
- Mesosystolic subocclusion of the LVOT by the anterior leaflet, VmaxLVOT > 2.5 m/s
- Cause: Restrictive annulus, excess valve tissue, hypovolemia, vasoplegia, stimulation β
- Treatment: Vasopressors, volume, stop amines, β blockers, surgical correction (5% of cases)
|
References
- BROWN ML, ABEL MD, CLOCK RL, et al. Systolic anterior motion after mitral valve repair: Is surgical intervention necessary? J Thorac Cardiovasc Surg 2007; 133:136-43
- CALAFIORE AM, GALLINA S, DI MAURO M, et al. Mitral valve procedure in dilated cardiomyopathy: repair or replacement? Ann Thorac Surg 2001; 71:1146-52
- arpentier AF, LESSANA A, RELLAND JYM, et al. The "Physio-Ring": an advanced concept of mitral valve annuloplasty. Ann Thorac Surg 1995; 60:1177-85
- CRESCENZI g, landoni g, zangrillo a, et al. Management and decision-making strategy for systolic anterior motion after mitral valve repair. J Thorac Cardiovasc Surg 2009; 137:320-5
- DE BONIS M, AL-ATTAR N, ANTUNES M, et al. Surgical and interventional management of mitral valve regurgitation: a position statement from the European Society of Cardiology working groups on Cardiovascular Surgery and Valvular Heart Disease. Eur Heart J 2016; 37:133-9
- DELOCHE A, JEBARA VA, RELLAND JY, et al. Valve repair with Carpentier techniques. The second decade. J Thorac Cardiovasc Surg 1990; 99:990-7
- ENDER J, EIBEL S, MUKHERJEE C, et al. Prediction of the annuloplasty ring size in patients undergoing mitral valve repair using real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr 2011; 12:445-53
- ESSANDOH M. Intraoperative echocardiographic assessment of mitral valve area after degenerative mitral valve repair: a call for guidelines or recommendations. J Cardiothorac Vasc Anesth 2016; 30:1364-8
- FISCHER GW, ANYANWU AC, ADAMS DH. Intraoperative classification of mitral valve dysfunction: The role of the anesthesiologist in mitral valve reconstruction. J Cardiothorac Vasc Anesth 2009; 23:531-43
- GILLINOV AM, COSGROVE DM, LYTLE BW, et al. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg 1997; 113:467-73
- GREWAL J, MANKAD S, FREEMAN WK. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009; 22:34-41
- KONGSAEREPONG V, SHIOTA M; GILLINOV AM, et al. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol 2006; 98:504-8.
- KWAK J, ANDRAWES M, GARVIN S, et al. 3D transesophageal echocardiography: a review of recent literature 2007-2009. Curr Opin Anesthesiol 2010; 23:80-8
- LANCELLOTTI P, TRIBOUILLOY C, HAGENDORFF A, et al. Recommendations for the echocardiographic assessment of native valvular regurugitation: an executive summary from the EACI. Eur Heart J Cardiovasc Imaging 2013; 14:611-44
- LEE APW, ACKER M, KUBO SH, et al. Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy. Circulation 2009; 119:2606-14
- MAHMOOD F, MATYAL R. A quantitative approach to the intraoperative echocardiographic assessment of the mitral valve for repair. Anesth Analg 2015; 121:34-58
- MANECKE GR, NGUYEN LC, TIBBLE AD, et al. Systolic anterior motion after mitral valve repair and a systolic anterior motion tolerance test. J Cardiothorac Vasc Anesth 2010; 24:883-4
- MASLOW A. Mitral valve repair: an echocardiographic review: Part I. J Cardiothorac Vasc Anesth 2015; 29:156-77
- MASLOW A. Mitral valve repair: an echocardiographic review: Part II. J Cardiothorac Vasc Anesth 2015; 29:439-71
- MASLOW AD, HAERING JM, LEVINE RA, et al. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol 1999; 34:2096-104
- MESSAS E, POUZET B, TOUCHOT B, et al. Efficacy of chordal cutting to relieve chronic persistent ischemic mitral regurgitation. Circulation 2003; 108:II111-II5
- RYAN L, JACKSON B, PARISH L, et al. Quantification and localization of mitral valve tenting in ischemic mitral regurgitation using real-time three-dimensional echocardiography. Eur J Cardiothorac Surg 2007; 31:839-4
- SUGENG L, CHANDRA S, LANG RM. Three-dimensional echocardiography for assessment of mitral valve regurgitation. Curr Opin Cardiol 2009; 24:420-5
- YAMAUCHI T, TANIGUCHI K, KUKI S, et al. Evaluation of the mitral valve leaflet morphology after mitral valve reconstruction with a concept of "coaptation length index". J Card Surg 2005; 20:432-5