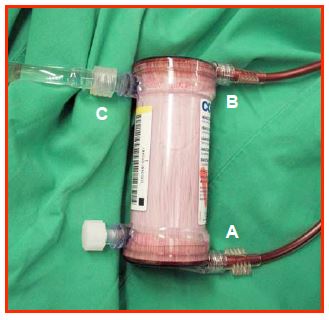
Haemofiltration is part of an ECC circuit consisting of semi-permeable microporous fibres contained in a cylinder (exchange surface ≥ 1 m2 ). This system filters water, electrolytes and the smallest proteins (molecular weight < 30-50 kDa) (Figure 7.14).
Figure 7.14: Haemofiltration system. The microporous filtration tubules inside the device can be spoted. A: Blood enters under pressure from the arterial circuit. B: outlet of the haemofiltered blood back to the venous circuit. C: wasted haemofiltrate.
The system is based on convection: hydrostatic pressure, not a difference in concentration, causes exchange across the membrane. Platelets, albumin and coagulation factors are retained, while water-soluble inflammatory mediators (TNF, IL-6, IL-8, IL-10) and complement (C3a, C5a) are removed with the haemofiltrate that is discarded [2,6]. The heparin is partially filtered. Different set-ups are possible (Figure 7.15).
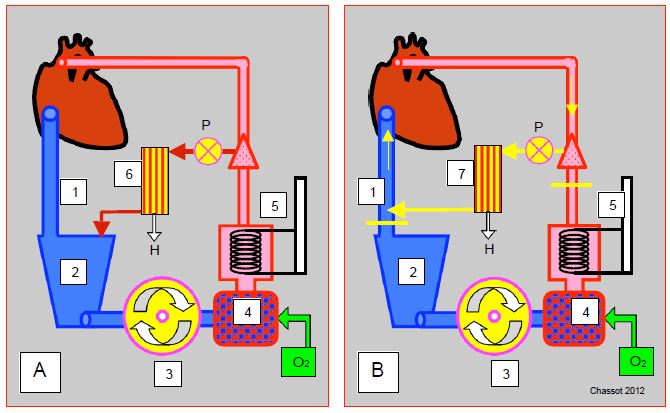
- Conventional ultrafiltration (CFU); blood is taken from the arterial line, usually with a pump, passes through the hemofilter and returns to the venous reservoir; the flow rate is adjusted so to maintain arterial flow back to the patient The system will only work properly if there is sufficient volume in the venous reservoir. Although it reduces the total volume of fluid , its effectiveness is limited by the redilution of the previously concentrated volume in the circuit.
- Continuous ultrafiltration (zero-balance UF: UFBZ); if continuous ultrafiltration is maintained to remove as many inflammatory triggers as possible, the large fluid volume that is being haemofiltered must be replaced by an equivalent supply to ensure adequate circulating volume. A dual-channel pump is fitted to the haemofiltrate outlet, which simultaneously ejects the haemofiltrate and replenishes it in a 1:1 ratio. This system is mainly used in paediatrics [3].
- Modified ultrafiltration (MUF) started after the end of the bypass. The blood is taken from the arterial line (aortic cannula), sucked by the UF circuit pump (15-30 mL/kg/min), hemofiltered and returned to the venous cannula from where it returns to the RA, since the venous cannula to the reservoir is clamped. The blood circuit is reduced to: aorta - UF pump - UF machine - venous cannula -RA. The rest of the bypass (reservoir, master pump, oxygenator, etc) is off. UFM delays decanulation and protamine administration by an average of about 20 minutes, and requires a lot of attention to maintain the filling of the circuit and the patient's blood volume [5].
Figure 7.15: Diagram of different haemofiltration systems. A: Conventional haemofiltration during ECC. Blood is collected from the arterial line (with or without P-pump), passes through the haemofilter (6) and returns to the venous reservoir (2); H: rejected haemofiltrate. B: post-ECC modified haemofiltration (MUF modified ultrafiltration). Blood is collected from the arterial line (aortic cannula), hemofiltered (7) and returned to the venous cannula from where it returns to the RA. Two clamps (yellow lines) isolate this circuit from the venous reservoir, the pump and the oxygenator. The P-pump is optional. 1: Venous cannula. 2: Venous reservoir. 3: Main pump. 4: Oxygenator. 5: Heat exchanger. 6: Conventional haemofiltration during ECC. 7: modified arteriovenous hemofiltration. H: rejected haemofiltrate. P: pump feeding the haemofiltrator.
The advantage of the procedure is that it removes excess fluid as a diuretic would, and restores the patient's haematocrit. The system can remove up to 180 mL per minute (4-5 L H2 O per hour) from the circulating volume at a flow rate of 500 mL/min. Some of the infusates used to compensate for fluid losses contain high levels of lactate (Lactosol® ); it is normal for the patient's lactate level to be elevated at the end of the bypass procedure without representing metabolic acidosis.
Haemofiltration corrects two major drawbacks of ECC : excess fluid and inflammatory syndrome. The preferred indications are situations where the patient's capacity for water elimination is reduced, and situations where the accumulation of water and Na+ is significant: long ECC, very positive water balance at the end of ECC, congestive heart failure, renal failure, lowered pulmonary functions, patients with excess fluid, and young children. The time to extubation of these patients, their blood loss and alveolar-capillary gradient are greatly improved by haemofiltration [3]. However, haemofiltration is increasingly seen as effective in reducing the burden of inflammatory triggers, decreasing the rate of transfusions and slowing the development of postoperative coagulopathies, interstitial oedema, pulmonary hypertension and multi-organ failure [1,4,6].
| Hemofiltration |
|
In its various forms, haemofiltration reduces excess fluid specific to ECC and removes water-soluble inflammatory triggers. It reduces postoperative interstitial oedema, transfusion requirements, systemic inflammatory syndrome and multi-organ failure.
Compared to blood washing systems (CellSaver™) that remove them, it retains platelets, heparin, plasma proteins and much of the clotting factors.
|
© CHASSOT PG, GRONCHI F, April 2008, last update December 2019
References
- BOODHWANI M, WILLIAMS K, BABAEV A, et al. Ultrafiltration reduces blood transfusion following cardiac surgery: a meta-analysis. Eur J Cardiothorac Surg 2006; 30:892-7
- JOURNOIS D, BOURDARIAS B. Hemofiltration in cardiac surgery. In: JANVIER G, LEHOT JJ (ed). Circulation extracorporelle: principes et pratique, 2nd edition. Paris, Arnette Groupe Liaison SA, 2004, 377-85
- JOURNOIS D, ISRAEL-BIET E, POUARD P, et al. High volume, zero-balance hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology 1996; 85:965-1003
- LUCIANI GB, MENON T, VECCHI B, et al. Modified ultrafiltration reduces morbidity after adult cardiac operations: a prospective randomized clinical study. Circulation 2001: 104:1253-9