Aortic valve replacement (AVR)
Surgical valve replacement, the only effective treatment for aortic stenosis in adults of all ages, is the second most common cardiac procedure after CABG. AVR immediately reduces LV wall stress. It is indicated in the following situations [1,4,38,53,57].
- Symptomatic narrow stenosis with area < 0.6 cm2 /m2, Vmax > 4 m/s (or > 4 times LVOT), mean gradient > 40 mmHg and peak gradient > 90 mmHg;
- Tight stenosis and ventricular dysfunction (EF < 0.50) regardless of symptoms;
- Symptomatic or non-symptomatic narrowing in patients undergoing bypass surgery for coronary artery disease or other valvular disease.
A mean gradient > 60 mmHg and a Vmax > 5 m/s are factors for spontaneous mortality that increase the indication for surgery [57]. In asymptomatic patients with valve area < 0.6 cm2 /m2 , AVR is not routinely indicated, but is reasonable in a number of circumstances [4,10,19,38,53].
- The dobutamine stress test induces symptoms of decompensation or an abnormal blood pressure response (50% of cases);
- The stress test increases the gradient if it is low and does not correspond to the degree of stenosis;
- The LV is dysfunctional (EF < 50%) and progressively dilated;
- Vmax increases > 0.3 m/s per year or is > 5 m/s (mean gradient > 60 mmHg);
- The valve is heavily calcified (high calcification index on multi-slice CT scan).
However, the risks of surgery outweigh the benefits in asymptomatic patients with normal exercise ventricular function or when severe comorbidities increase the risk of surgery [38]. In these circumstances, active surveillance is recommended. Surgery should be indicated at the first sign of deterioration, as the mortality rate of expectant management is 3-4 times higher than that of surgical correction at the onset of symptoms or ventricular dysfunction [19].
The operative mortality rate is 1-3% for simple cases and 3-6% for complex procedures (associated CABG, Bentall operation, etc) [12,27]. Although not a contraindication to intervention, ventricular failure is the main risk factor: mortality is up to 15% in cases of left ventricular failure. The best criterion for assessing true LV function is the value of end-diastolic dimensions; dysfunction is characterised by an LV short-axis diameter > 4 cm2 /m2 .
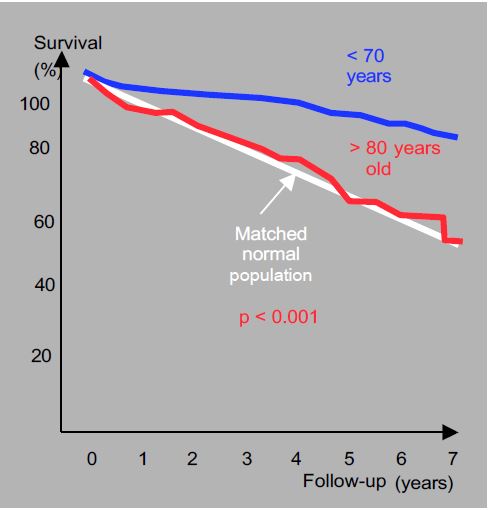
In the elderly, current results show that it is never too late to intervene in symptomatic patients, as their functional recovery is remarkable, although the operative mortality rate in octogenarians is 9% [6,24,44,53]. In addition, the progression of stenosis after the age of 80 is twice as fast as in younger patients [30]. Although the 5-year survival rate of octogenarians (66%) is lower than that of patients aged <70 years (86%), it is superimposed on the life expectancy of healthy individuals of the same age (Figure 11.119) [16]. The operation therefore puts the patient back on the physiological survival curve (see Chapter 21 Cardiac Surgery in the Elderly Patient). However, valve replacement, even by TAVI, is not indicated if life expectancy is < 1 year [57].
Figure 11.119: Long-term survival of octogenarians undergoing aortic valve replacement for tight stenosis [16]. Although 5-year survival is lower than in patients aged <70 years, it is superimposed on the life expectancy of healthy individuals of the same age. AVR is therefore justified in symptomatic elderly patients with a life expectancy of several years, as it ensures normal survival for their age.
In the case of LV dysfunction (EF < 50%), the gradient may be deceptively low (low-flow/low-gradient situation): only the stress test (dobutamine) can tell the difference between a narrow stenosis decompensating the LV (indication for AVR) and cardiomyopathy with simple valvular fibrosis (pseudostenosis with no indication for surgery) (see Nosology). If the test reveals some contractile reserve, the mortality rate for AVR is 4-7%; if this reserve is absent, the mortality rate is 22% [51]. However, even in the latter case, surgery offers better long-term survival than medical treatment (65% versus 11% at 5 years) [51]; given the operative risk, percutaneous transluminal angioplasty (TAVI) is preferred in this situation. AVR may be urgently indicated in cases of acute left-sided decompensation when aortic stenosis is the determining factor. As operative mortality in this setting is high (>20%), an infusion of nitroprusside in the short term may exceptionally be a less dangerous solution to reduce the patient's afterload and allow survival to surgery under good conditions [26].
The excess afterload caused by aortic stenosis increases the importance of mitral regurgitation, if present. In the presence of normal mitral leaflets and in the absence of annular dilatation or ventricular deformation, MI is likely to resolve after AVR [1].
Deaths are mainly due to heart failure or infarction. Mortality directly related to the prosthesis accounts for only 20% of cases: thromboembolism, endocarditis, bioprosthesis degeneration, mechanical dysfunction or paravalvular leakage [27]. The presence of significant coronary artery disease worsens the postoperative prognosis of isolated aortic valve replacement (AVR), but the addition of AVR to CABG does not worsen perioperative mortality in cases of ischaemic disease; there is therefore every reason to be aggressive in the indication for surgery.
In the absence of associated risk factors, the postoperative course is surprisingly simple: removal of the obstruction provides immediate relief to the LV. Provided the size of the prosthesis is appropriate for the patient, the reduction in afterload following surgical correction leads to immediate clinical improvement and significant subsequent recovery; the majority of patients fall into NYHA class I-II. Patients with pre-operative congestive failure are subjectively improved despite little change in systolic function indices (ejection fraction) and ventricular morphology (hypertrophy and dilatation) [23]. Ventricular compliance and distensibility tend to improve in the long term after valve replacement, as hypertrophy regresses during the first 3 months [55]. Anatomical recovery depends on the extent of preoperative degenerative changes. If LV end-diastolic pressure is still normal at the time of surgery, ventricular hypertrophy regresses significantly for up to one year after surgery [36]; however, if LV end-diastolic pressure was high or contractility was reduced preoperatively, the reduction in ventricular mass is only partial. It is therefore preferable to intervene before the decompensation phase, but the indication for surgery remains very broad, even when the patient is in congestive failure, as clinical improvement is always possible.
Patient-prosthesis mismatch occurs when the effective surface area of the prosthesis is too small in relation to the patient's height. It can be moderate (S 0.65 - 0.85 cm2 /m2 ) or severe (< 0.6 cm2 /m2 ) (see Complications of prostheses, Patient-prosthesis mismatch). This mismatch results in an excessive transvalvular gradient and adversely affects the patient's outcome. When severe, it leads to persistent ventricular hypertrophy, long-term worsening of LV dysfunction and an increase in 5-year mortality of up to 15-25% [2,45]. The incidence of severe mismatch is 9-12% and moderate mismatch is 30-50% [3,20]. The clinical impact is significant in young people with high cardiac output, but less pronounced in those aged >70 years [15]. A significant patient-prosthesis mismatch in an elderly patient is unlikely to justify a return to ECC to try to fit a larger valve.
Complete AV block may occur due to the proximity of the aortic annulus to the His bundle at the level of the interventricular septum: attachment points of the prosthesis may damage the conducting structures. As AVR is often performed in elderly patients with highly calcified valves and vessels, the post-operative stroke rate is 3-6%, i.e. 2-3 times higher than average.
Overall survival at 10 years is 75-90% [28]. In patients aged <55 years, whose survival is independent of age-related comorbidities, perioperative mortality after mechanical AVR is 1-3% and annual mortality 1.55%/year; the average life expectancy of a mechanical aortic prosthesis recipient is 19 years, half that of a person in their forties. Anticoagulation-related complications are 0.85%/year and the reoperation rate is 0.5%/year [29]. With a bioprosthesis, a 40-year-old patient has a life expectancy without reoperation of 38% at 20 years [8].
Percutaneous valvulotomy
In cases where the valve is neither calcified nor insufficient (tight biscuspidia or monocuspidia in children and adolescents), percutaneous dilatational valvulotomy can be performed using a balloon guided by catheterisation, a minimally invasive procedure that avoids the need for prosthesis implantation [13]. The technique gives good results in children and adolescents when the gradient is > 60 mmHg and there is no insufficiency: 50% of cases have no recurrence at 8 years [35]; a good indication is non-calcified tight stenosis before pregnancy. In older adults, however, the technique is of limited value due to calcification and the risk of embolism if the valve fractures; long-term results are disappointing: 50% restenosis at 6 months [18,41]. However, valvulotomy may be indicated in patients at high surgical risk requiring emergency intervention or palliation: cardiogenic shock with decompensated stenosis, critical stenosis during pregnancy, or emergency non-cardiac surgery [37,38,53]. This technique makes it possible to temporarily increase the useful surface area of the valve by 0.5 cm2 and significantly reduce the gradient (reduction in ΔPmean of 25 mmHg) while awaiting definitive surgery, but the risks are high (20% of cases): embolism of calcified particles with neurological deficits, severe aortic insufficiency, myocardial infarction [22]. Percutaneous valvulotomy is not a substitute for surgical valve replacement. In adults, it is currently being replaced by transcatheter aortic valve implantation (TAVI).
AVR by endovascular prosthesis (TAVI)
A more promising technique is transcatheter aortic valve implantation (TAVI) (see Chapter 10, TAVI). Several types of aortic valve are in clinical use and others are being tested (see Figure 11.40). These are biological valves that are inserted into a self-expanding prosthesis or expanded by inflating a balloon; they are placed via the femoral or transapical route (left mini-thoracotomy). They have an effective surface area of approximately 1.7 cm2 and a gradient of 10-12 mmHg [46]. This technique is preferred in situations where the risk of surgical AVR is high (>8%) and the mortality of AVR is prohibitive (>20%), particularly in the elderly, who have limited survival [1]. It is also preferred in technically challenging conditions such as porcelain aorta, reintervention or thoracic irradiation.
TAVI is currently superior to AVR in high-risk patients and non-inferior (or even superior if the transfemoral route is available) in intermediate-risk patients. Compared with AVR, the main complications of TAVI are vascular lesions, paravalvular leaks and the rate of permanent pacemakers [31]. The risk of stroke is similar. The rates of atrial fibrillation, renal failure and bleeding are lower than with surgery [1,32,50].
In-hospital mortality is 2-9%; 1-year survival is 70-80% [14,31]. The popularity of transcatheter AVR and continuous improvements in the technology mean that more than 300,000 patients have been treated with this procedure to date. The evidence that TAVI is equivalent to conventional AVR provided by the PARTNER trial extends the indications to less sick and younger populations [48]. Interim results already show that in moderate-risk patients aged > 65 years operated in experienced centres, the 1-month mortality of TAVI is 1.1-4.4%, which is encouraging and supports the comparison with the mortality of conventional AVR, which is 2.4% > 75 years and 1.3% in patients aged < 70 years [33]. Long-term follow-up is not available, as the longest follow-up for TAVI is 8 years [7], but in an experiment simulating chronic fatigue for bioprostheses, the durability of TAVI is 7.8 years and that of AVR is 16 years [34].
TAVI could be a solution in the specific situation of moderate aortic stenosis (1.0-1.5 cm2 ) accompanied by significant ventricular dysfunction, because this configuration is dangerous and deterioration is rapid: at 4 years, death or left-sided failure occurs in 60% of these patients [55]. Reducing afterload with a non-invasively implanted prosthesis is likely to be an option that increases survival and slows ventricular decompensation.
AVR and narrow aortic annulus
Between 15% and 20% of patients with aortic stenosis have a narrow valve annulus (< 15 mm/m body height), which can accommodate prostheses < 21 mm in diameter. Postoperatively, half of these patients suffer from a patient-prosthesis mismatch (opening area < 0.85 cm2 /m2 ) (see Complications of heart valve prostheses). A number of interventions are available for these patients [17].
- Mounted bioprosthesis; the least restrictive are the Mitroflow™ and Trifecta™ models, which have the lowest gradients.
- Bioprosthesis without a frame (stentless); the thinness and flexibility of these valves (Freedom Solo™, Freestyle™) allow prostheses to be implanted that are 3-4 mm wider than previous ones.
- Bioprosthesis without fixation (sutureless); implantation in ECC of a prosthesis by self-expansion or balloon inflation (Perceval™, Intuity™), similar to percutaneous prostheses, reduces operative time and allows placement of a larger prosthesis as the native valve is excised.
- Aortic ring dilatation; although this prolongs the operation and increases the risk of atrioventricular block, it increases the size of the implantable prosthesis and reduces the risk of patient-prosthesis mismatch.
- TAVI (transcatheter aortic valve implantation); the gradients of these percutaneous valves are lower than those of a conventional bioprosthesis for the same size, but the risk of aortic insufficiency due to paravalvular leakage is significant.
- Ross procedure; transposition of the pulmonary valve to the aortic position and placement of a homograft or heterograft in the pulmonary position; in young patients, this complex and difficult technique allows the valve to grow.
In patients at low surgical risk, AVR with a Mitroflow™, Trifecta™ or rimless bioprosthesis is the first choice; if the risk of mismatch is high, a rimless prosthesis or enlargement surgery may be considered. In patients with intermediate or high surgical risk, TAVI is preferred because it provides better haemodynamic results [17]. The aortic annulus is oval in shape; two-dimensional echocardiography slices it at its smallest diameter and therefore tends to underestimate the true open area. 3D imaging, on the other hand, allows accurate measurement and is essential for assessing the risk of patient-prosthesis mismatch.
AVR and other cardiac procedures
As coronary artery bypass grafting (CABG) and aortic valve replacement (AVR) are the two most common procedures in cardiac surgery, it is not uncommon for them to be combined. Combining the two procedures has an operative mortality of 5% to 15%, depending on age [38]. However, not adding CABG to AVR in an ischaemic patient or leaving aortic stenosis in a patient with coronary artery disease who has undergone surgery has a much worse postoperative prognosis [53]. Even if the stenosis is moderate (S = 1.0-1.5 cm2 ), it is reasonable to replace the aortic valve at the time of CABG because the operative mortality of a subsequent AVR is an additional operative risk that is greater than that of combined surgery [4,43].
In the case of tight bicuspid stenosis, the presence of ascending aortic dilatation requires replacement of the aorta if its diameter is ≥ 4.5 cm in a symptomatic patient and ≥ 5.5 cm in an asymptomatic patient (see Aortic bicuspidity) [21].
The presence of narrow aortic stenosis at the time of mitral valve surgery justifies concomitant AVR [4]. Conversely, the presence of significant mitral insufficiency (MI) in a patient undergoing aortic valve replacement occurs in 20% of AVRs and poses the problem of simultaneous correction of the two pathologies, resulting in an increase in operative mortality: on average 2.7% for single AVR and 6.5% for double replacement [49]. It is important for the anaesthesiologist to define the mechanism and severity of the MI as accurately as possible in order to make an optimal surgical decision [39].
- Severe MI: Regurgitant orifice > 0.4 cm2 and vena contracta > 0.7 cm in structural MI (> 0.3 cm2 and > 0.4 cm in ischaemic MI) are the most relevant indices. Colour jet size, PISA and regurgitation fraction are too dependent on haemodynamic conditions (increased LV afterload due to aortic stenosis). The presence of severe MI is generally accepted as an indication for simultaneous replacement (MVR) or plasty (MVP), as it triples the mortality of AVR and the risk of cardiogenic shock during perioperative haemodynamic changes [52].
- Moderate or moderate to severe organic MI (regurgitant orifice 0.2-0.4 cm2 and vena contracta 0.3-0.6 cm): although it increases operative mortality, it is not a formal indication for mitral valve surgery; it depends on the context.
- Functional MI: With reduction of intraventricular pressure, MI regresses after AVR in half of the cases, but does not improve in the long term in the other half [54].
- Factors favouring regression of MI after AVR: functional MI due to LV dysfunction, high preoperative transvalvular pressure gradient.
- Factors associated with non-regression of MI after AVR: mitral annular calcification, degenerative MI, dilatation of the LA, chronic AF, pulmonary hypertension.
| Indications and surgical outcomes |
|
Immediate and definitive relief of aortic obstruction improves LV function; the excellent results of aortic valve replacement (AVR) mean that the indications are broad, irrespective of age. Operative indications for AVR:
- Tight stenosis (area < 0.6 cm2 /m2 ) in a symptomatic patient
- Tight stenosis and ventricular dysfunction (EF < 0.5)
- Tight stenosis associated with other bypass surgery (CABG, MVR, MVP, aorta)
- Asymptomatic patient: only if stress test abnormal, severely calcified valve or dilatation
Progressive stenosis of the LV (LV Dtd > 4.0 cm/m2, EF < 50%) in a tight stenosis
Mild or moderate stenosis: no evidence. The intensity of symptoms associated with aortic stenosis is the main indication for surgery.
Mortality of AVR: 1-3% for simple cases, 4-7% for complex procedures.
Survival at 10 years: 85-90%. In patients > 80 years of age: 66% (= pathology-free life expectancy).
Indications for percutaneous valvulotomy (recurrence rate: 50% at 6 months in adults):
- Patients with non-calcified or insufficient valve, too young for AVR
- Decompensated stenosis in pregnancy
- Rescue in cardiogenic shock or preoperative vital non-cardiac surgery
Indications for bioprosthesis implantation (TAVI) :
- Patients at high risk (>8%) or prohibitive (>20%) for AVR
- Technically challenging situations (porcelain aorta, thoracic irradiation, etc.)
- Intermediate risk patients > 65 years of age
Operative mortality: 2-7%; 1-year survival: 70-80%.
|
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BLAIS C, DUMESNIL JG, BAILLOT R, et al. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation 2003; 108:983-8
- BLEIZIFFER S, EICHINGER WB, HETTICH I, et al. Impact of patient-prosthesis mismatch on exercise capacity in patients after bioprosthetic aortic valve replacement. Heart 2008; 94:637-41
- BONOW RO, BROWN AS, GILLAM LD, et al. ACC/AATS/AHA/ASE/EACTS/HVS/SCA/SCAI/SCCT/SCMR/STS/ 2017 appropriate use criteria for the treatment of patients with severe aortic stenosis. J Am Coll Cardiol 2017; 70:2566-98
- BONOW RO, CARABELLO B, KARU C, et al. ACC/AHA guidelines for the management of patients with valvular heart disease: Executive summary. Circulation 2006; 114:284-91
- BOSE AK, AITCHISON JD, DARK JH. Aortic valve replacement in octogenarians. J Cradiothorac Surg 2007; 2:33
- BOULETI C, HIMBERT D, IUNG B, et al. Long-term outcome after transcatheter aortic valve implantation. Heart 2015; 101:936-42
- BOURGUIGNON T, EL KHOURY R, CANDOLFI P, et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 60 or younger. Ann Thorac Surg 2015; 100:853-9
- COWELL SJ, NEWBY DE, PRESCOTT RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352:2389-97
- DAL-BIANCO JP, KHANDHERIRA BK, MOOKADAM F, et al. Management of asymptomatic severe aortic stenosis. J Am Coll Cardiol 2008; 52:1279-92
- DICHTL W, ALBER HF, FEUCHTNER GM, et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am J Cardiol 2008; 102:743-8
- EDWARDS FH, PETERSON ED, COOMBS LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol 2001; 37:885-94
- ELLIOTT JM, TUZCU EM. Recent developments in balloon valvuloplasty techniques. Curr Opin Cardiol 1995; 10:128-33
- FASSL J, AUGOUSTIDES JGT. Transcatheter aortic valve implantation – Part 1: Development and status of the procedure. J Cardiothorac Vasc Anesth 2010; 24:4
- FEINDEL CM. Counterpoint: aortic valve replacement: size does matter. J Thorac Cardiovasc Surg 2009; 137:284-5
- FILSOUFI F, RAHMANIAN PB, CASTILLO JG, et al. Excellent early and late outcomes of aortic valve replacement in people aged 80 and older. J Am Geriatr Soc 2008; 56:255-6198-505
- FREITAS-FERRAZ A, TIRADO-CONTE G, DAGENAIS F, et al. Aortic stenosis and small aortic annulus. Clinical challenges and current therapeutic alternatives. Circulation 2019; 139:2685-702
- GALAL O, RAO PS, AL-FADLEY F, WILSON AD. Follow-up result of balloon aortic valvuloplasty in children with special reference to causes of late aortic insufficiency. Am Heart J 1997; 133:418-35
- GÉNÉREUX P, STONE GW, O'GARA PT, et al. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2016; 67:2263-88
- HEAD SJ, MOKHLES MM, OSNABRUGGE RI, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27'186 patients with 133'141 patient-years. Eur Heart J 2012; 33:158-29
- HIRATZKA LF, CREAGER MA, ISSELBACHER EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves. J Am Coll Cardiol 2016; 67:724-31
- HOLMES DR, NISHIMURA DA, REEDER GS. In-hospital mortality after balloon aortic valvuloplasty: Frequency and associated factors. J Am Coll Cardiol 1991; 17:189-94
- HWANG MH, HAMMERMEISTER KE, OPRIAN C, et al. Preoperative identification of patients likely to have left ventricular dysfunction after aortic valve replacement. Circulation 1989; 80(suppl I):I-65
- IUNG B, CACHIER A, BARON G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery ? Eur Heart J 2005; 26:2714-20
- IUNG B, GOHLKE-BÄRWOLF C, TORNOS P, et al. Recommandations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J 2002; 23:1253-66
- KHOT UN, NOVARO GM, POPOVIC ZB, et al. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N Engl J Med 2003; 348:1756-63
- KIRKLIN JW, BARRATT-BOYES BG. Aortic valve disease. In: KIRKLIN JW. Cardiac surgery. New York, Churchill- Livingstone, 1993, 491-571
- KIVDAL P, BERGSTRÖM R, HÖRTE LG, etal. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000; 35:747-53
- KORTELAND NM, ETNEL JRG, ARABKHANI B, et al. Mechanical aortic valve replacement in non-elderly adults: meta-analysis and microsimulation. Eur Heart J 2017; 38:3370-7
- KUME T, KAWAMOTO T, OKURA H, et al. Rapide progression of mild to moderate aortic stenosis in patients older than 80 years. J Am Soc Echocardiogr 2007; 20:1243-6
- LEON MB, SMITH CR, MACK M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363:1597-607
- LEON MB, SMITH CR, MACK M, et al, for the PARTNER 2 investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609-20
- LINKE A, WENAWESER P, GERCKENS U, et al. Treatment of aortic stenosis with a self-expanding transcatheter valve: the international multicentre ADVANCE study. Eur Heart J 2014; 35:2672-84
- MARTIN C, SUN W. Comparison of transcatheter aortic valve and surgical bioprosthetic valve durability: a fatigue simulation study. J Biomech 2015; 48:3026-34
- McCRINDLE BW. Independent predictors of immediate results of percutaneous balloon aortic valvotomy in children. Am J Cardiol 1996; 77:286-93
- MONRAD ES, HESS OM, MURAKAMI T, et al. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation 1988; 77:1345
- MORENO PR, JANG IK, NEWELL JB, et al. The role of percutaneous aortic balloon valvuloplasty in patients with cardiogenic shock and critical aortic stenosis. J Am Coll Cardiol 1994; 23:1071-7
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- NOMBELA-FRANCO L, BARBOSA RIBEIRO H, URENA M, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement. J Am Coll Cardiol 2014; 63:2643-58
- OTTO CM, BURWASH IG, LEGGET ME, et al. Prospective study of asymptomatic valvular aortic stenosis: Clinical, echocardiographic and exercise predictors of outcome. Circulation 1997; 95:2262-70
- OTTO CM, MISKEL MC, KENNEDY JW, et al. Three years outcome after balloon aortic valvuloplasty: Insights into prognosis of aortic valvular stenosis. Circulation 1994; 90:II-205
- PALLIKKA PA, NISHIMURA RA, BAILEY KR, TAJIK AJ. The natural history of adults with asymptomatic hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15:1012-9
- PERREIRA JJ, BALABAN K, LAUER MS, et al. Aortic valve replacement in patients with mild or moderate aortic stenosis and coronary bypass surgery. Am J Med 2005; 118:735-42
- PETER M, HOFMAN A, PARKER C, et al. Progression of aortic stenosis: Role of age and concommittant coronary artery disease. Chest 1993; 103:1715-9
- RAHIMTOOLA SH. Choice of prosthetic heart valve in adults. An update. J Am Coll Cardiol 2010; 55:2413-26
- SELLEVOLD OFM, GUARRACINO F. Transcutaneous aortic valve implantation: recent advances and future. Curr Opin Anaesthesiol 2010; 23:67-73
- SHAVELLE DM, TAKASU J, BUDOFF MJ, et al. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet 2002; 359:1125-8
- SMITH G, LEON MB, MACK MJ, et al, for the PARTNER Trial Investigators. Transcatheter versus surgical aortic valve replacement in high risk patients. N Engl J Med 2011; 364:2187-98
- STS – Society of Thoracic Surgeons National Cardiac Surgery Database, 2017. https://www.sts.org/site/defaut/files/documents/ ACSD_ExecutiveSummary2017Harvest4_RevisedReport.pdf
- THYREGOD HG, STEINBRUCHEL DA, IHLEMANN N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis : 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol 2015; 65:2184-94
- TRIBOUILLOY C, LEVY F, RUSINARU D, et al. Outcome after aortic valve replacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. J Am Coll Cardiol 2009; 53:1865-73
- UNGER P, CLAVEL MA, LINDMAN BR, et al. Pathophysiology and management of multivalvular disease. Nat Rev Cardiol 2016; 13:429-40
- VAHANIAN A, ALFIERI O, ANDREOTTI F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012; 33:2451-96
- VAN DEN EYDEN F, BOUCHARD D, EL-HAMAMSY I, et al. Effect of aortic valve replacement for aortic stenosis on severity of mitral regurgitation. Ann Thorac Surg 2007; 83:1279-84
- VITARELLI B, VASSALLI G, MONRAD ES, et al. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation 1995; 91:2353-8
- VON GILS L, CLAVEL MA, VOLLEMA EM, et al. Prognostic implications of moderate aortic stenosis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2017; 69:2383-92
- ZIMMERMAN J, BIRGENHEIER N. Appropriate Use Criteria for the treatment of patients with severe aortic stenosis: a review of the 2017 American College of Cardiology Guideline for the cardiac anesthesiologist. J Cardiothorac Vasc Anesth 2019; 33:3127-42