Extracorporeal circulation (ECC) has in fact quite a long history. The idea of an artificial perfusion is awarded to the French physiologist Jean-Jacques Le Gallois who, in 1812, perfused decapitated rabbit's heads to prove blood circulation maintained organ function. The heart-lung machine prototype including temperature control was devised in 1884 by von Frey and Gruber, but Hooker, in 1915, built the forerunner of film oxygenators: it consisted of a rubber disc on which the blood spread in an oxygenated film by direct contact with an O2 flow. These set-ups were not very successful, as the blood coagulated very quickly. Indeed, it was not until the discovery of heparin in 1916 and protamine 20 years later that the issue was solved [3]. In 1937 John Gibbon created the first complete ECC machine that allowed animals survival in laboratory; the oxygenator consisted of a rotating disc screen. In 1953, this machine allowed the successful closure of an ASD by a 18-year-old patient during a 45-minute bypass [1]. For Gibbon, it’s been the climax of 23 years of research and design in extracorporeal circuits. He became the "father of ECC" and the first perfusionist in history. At first, the road was paved with deaths (1 survivor out of 16 attempts), because the first ECC circuits were cumbersome and dangerous; they required a priming of several liters of blood, and operated with an oxygenator made of large discs partially immersed in blood and rotating in a chamber full of oxygen. The whole thing was washed and reused for another patient. Only tubings were single-use. Same for the first machine in Lausanne, the Livio-Mettraux model, dating from 1962 (Figure 7.1A).
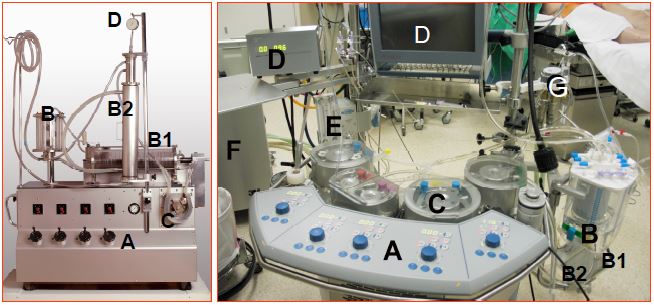
Figure 7.1: ECC machines. Left: historical Livio-Mettraux machine, Lausanne 1962. Right: Current machine. A: Central console with pump controls (blue buttons). B: venous reservoir. B1: oxygenator. B2: heat exchanger (coupled to the oxygenator and the reservoir in the modern machine). C: pumps. D: monitoring; simple pressure gauge on left, complex control screens on right. E: gas mixer and vaporiser. F: tank providing variable temperature water for the heat exchanger. G: Cardioplegia circuit. After more than 50 years, it is striking that the layout of the two machines is identical; the pumps have hardly changed, but the oxygenator has been completely transformed and the monitoring has become more sophisticated. In addition, all parts in contact with blood are now single-use.
The problem of the oxygenator remained nagging, as the disc models caused numerous problems and lacked efficiency. As early as 1956, DeWall and Lillehei designed a system that could be made of plastic and consisted of a chamber where the blood was oxygenated by bubbling oxygen, topped by a debubbling chamber filled with an anti-foaming agent and a spiral tank. New companies began marketing these devices: Bentley™, Travenol™, etc. The first device widely used in Europe was the Rygg and Kyvsgaard model; it consisted of a disposable polyethylene bag containing all the components; it’s been widely used until the late 1970s because it was simple, effective and cheap (see Figure 1.3) [2]. However, the bubble oxygenator was still a major cause of gas embolism; moreover, as soon as ECC exceeded one hour, direct contact of blood with air led to protein denaturation and complement activation, which caused a massive systemic inflammatory syndrome and very often ARDS, also known as pump lung. As renal dialysis membranes were efficient, the idea emerged to use them for O2 diffusion without direct contact with the blood. Plates of ethyl cellulose, Teflon™ and then silicone and polypropylene, arranged in layers or tubules, were used; the most recent models are made of poly-methyl-pentene. Although the problems of air-blood contact have been solved, membrane oxygenators have been slow to catch on because of their complexity and price. Today, their sophistication and reliability explain why they are the only systems used in ECC machines; they consist of a unit including an oxygenator, venous reservoir and heat exchanger (see Figure 7.6).
With the exception of the pump itself, all components of the bypass machine are now single-use. The circuit also includes many safety systems that were not part of the early models: bubble monitor, arterial and venous filters, SaO2 and SvO2, servo control, of the reservoir level by pump, etc. Current work focuses on improving the biocompatibility of contact surfaces, reducing the inflammatory response, downsizing the whole system.
Despite the development of highly qualified training for perfusionists, national certificates of competence and realistic simulators, the conduct of ECC is a very small-scale discipline where the main teaching takes place on the field. Furthermore, there are no high-level evidence-based recommendations for artificial perfusion, temperature management or organ protection [2]. After 65 years of existence, ECC remains an area of institutional routines governed by the precautionary principle and manufacturers' prescriptions rather than by scientific data and well-controlled studies. Although complicated, mechanical systems imitating cardio-respiratory functions remain crude compared to the subtlety of physiological regulation. Given the infinitely complex interdependence of the various factors involved, it is almost illusory to look for simple correlations between postoperative morbidity and isolated data such as MAP or flow. On the other hand, ensuring circulation and ventilation through a machine, however sophisticated, remains a totally non-physiological situation and therefore is a huge stress for the body. It is the equivalent of a stay in a hostile environment, such as mountaineering at high altitude or scuba diving: the body hardly survives it for long. Consequences are numerous : trigger of inflammatory response, coagulation disorders, immunomodulation, haemodynamic disturbances, altered gas exchange, polyorganic dysfunction.
| Technology evolution |
| The first clinical ECC dates back to 1953. At that time, the oxygenator was made of a series of rotating discs on which blood was spread in contact with O2. This system has been replaced by bubble oxygenators, and currently by membrane oxygenators. All blood-contacting components are now single-use (cannulae, tubing, reservoir, oxygenator, heat exchanger, filters) and designed for ease of handling.
Despite its refinements and daily clinical success, ECC is a non-physiological situation that causes a huge stress response and cascade of inflammatory, coagulative and polyorganic complications. |
© CHASSOT PG, GRONCHI F, last update December 2019
References
- GIBBON JH. Application of the mechanical heart and lung apparatus to cardiac surgery. Minn Med 1954; 36:171-85
- HESSEL EA. History of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 2015; 29:99-111
- STAMMERS AH. Historical aspects of cardiopulmonary bypass: From antiquity to acceptance. J Cardiothorac Vasc Anesth 1997; 11:266-74