The three direct factor Xa inhibitors currently on the European market are oral substances that require 1-2 doses per day, but do not require coagulation monitoring (see Tables 8.1, 8.2 and 8.3). A series of large multi-centre randomised trials have clarified the clinical indications for these new drugs [15,33]. The times for discontinuation of these drugs prior to surgery are outlined under Perioperative Management (see Tables 8.7A and 8.7B, 8.11 and 8.12).
Rivaroxaban (Xarelto® )
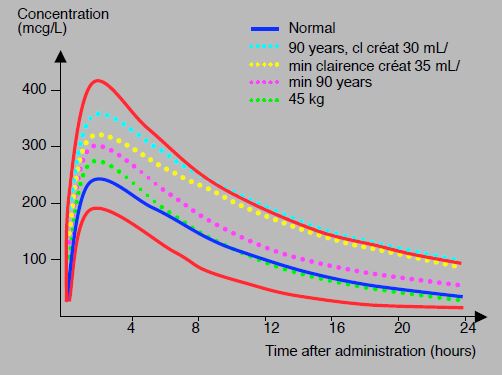
Rivaroxaban is a direct competitive inhibitor of the factor Xa active site. After oral ingestion, bioavailability is 80%, and peak concentration is reached in 2.5-4 hours. The half-life is 5-9 hours, but 11-13 hours > 75 years and in renal failure. Protein binding is very high (92-95% of circulating substance). Elimination is by hepatic metabolism for two thirds and by renal excretion for one third [5]. Serum levels obtained with a 20 mg dose average 250 ng/mL at peak concentration (2-4 hours after dosing) and 40-50 ng/mL at nadir (12-24 hours); they are 45 and 16 ng/mL respectively with the 2 times 2.5 mg/d dose [27,30]. However, the values obtained with the same dosage show a large inter-individual variability (47-93%) [30], which is related to age and renal function (see Figure 8.11A) [26].
Although factor Xa inhibition is directly proportional to plasma level of the substance, there are currently no data on the relationship between plasma level and bleeding risk [27]. However, it seems logical to assume that they increase in parallel. As with all other xabans, there may be drug interactions with other substances whose metabolism is also dependent on hepatic cytochromes CYP3A4 and P-glycoprotein transport. For example, azole antifungals, anti-HIV proteases, some antibiotics (clarithromycin) and some antiarrhythmics (amiodarone, verapamil, dronedarone) increase rivaroxaban levels by 2.5-fold, while phenobarbital, carbamazepine, phenytoin, rifampicin and St John's wort decrease them by 50% [34]. There is no interaction with statins [18,19]. Rivaroxaban significantly prolongs PT and non-significantly prolongs aPTT. PT cannot be used to quantify the effect. A normal PT does not rule out rivaroxaban; however, it indicates that the patient is unlikely to have high levels of the drug. The specific test is the calibrated anti-Xa activity for rivaroxaban (see Monitoring and Table 8.4) [1,12,15]. Rivaroxaban is contraindicated in pregnant women, during lactation, and in patients with impaired liver (Child-Pugh B and C) or renal function (creatinine Cl < 30 mL/min) [1].
Preoperatively, the waiting time for surgical procedures depends on several factors (see Table 8.12) [2,18,23,36].
Figure 8.11A: Concentration profiles of rivaroxaban (20 mg 1 x/d) versus time in different situations: 90-year-old patients with renal failure (turquoise dotted line), patients with creatinine clearance of 35 mL/kg (yellow dotted line), 90-year-old patients (purple dotted line), 45 kg patients (green dotted line). The curve of a standard individual is shown in blue. In red, curves for the 5th and 95th percentiles; the upper value is about 3 times the lower value [modified from ref 26].
- 24 hours for simple surgery in patients without comorbidities if the dosage is 5 or 10 mg/d;
- 48 hours for surgery without risk of bleeding if the dosage is 15-20 mg/d;
- 72 hours for operations with high bleeding risk and for spinal LRA;
- 72-120 hours if creatinine clearance is < 50 mL/min (depending on other risk factors).
If the Clcréat is < 30 mL/min, the substance is contraindicated. For spinal loco-regional anaesthesia (epidural, intrathecal) or deep blocks (including periclavicular blocks), it is recommended to wait 72 hours for safety [18]. Removal of the epidural catheter should be delayed until 48 hours after the last dose. A delay of 6 hours should be observed between puncture or removal of the epidural catheter and resumption of the drug with low doses; this is increased to 12 hours with high doses [31].
In Europe, rivaroxaban has one third of the anticoagulant market and three quarters of the NOAC market. It is accepted for the following indications:
- Prophylaxis of postoperative deep vein thrombosis (10 mg/d), started 6-12 hours after surgery [37]; DVT prophylaxis in bedridden or medically high-risk patients [35].
- Treatment of acute deep vein thrombosis (DVT) and pulmonary embolism (15 mg 2x/d for 3 weeks, then 20 mg/d) [6].
- Prevention of systemic embolism (stroke) in non-valvular atrial fibrillation (20 mg/d) [28].
- Treatment of acute coronary syndrome as an adjunct to aspirin and clopidogrel (2.5 mg 2x/d) [25].
- Treatment of stable coronary artery disease in combination with aspirin (2.5 mg 2x/d) [14].
- Anticoagulation for AF in patients on antiplatelet therapy for angioplasty and coronary stenting (15 mg/d + clopidogrel alone, or 5 mg/d + aspirin + clopidogrel) [16,24].
Apixaban (Eliquis® )
Apixaban is also a direct competitive inhibitor of factor Xa. It has a half-life of 8-15 hours, with three quarters of elimination by hepatic metabolism and one quarter by renal excretion [5]. As with rivaroxaban, drug interference is possible with substances whose metabolism is dependent on the liver cytochromes CYP3A4. It should also be avoided in pregnant women and in patients with hepatic or renal insufficiency (creatinine Cl < 30 mL/min) [1]. Apixaban (2.5-5 mg 2x/d) unreliably prolongs PT and has little effect on aPTT [12,15]. The test of choice is anti-Xa activity. Preoperatively, it is recommended to wait 48 hours after the last dose before proceeding with surgery and, to be safe, 72 hours for spinal LRA. This is extended to 3-4 days if the patient is > 75 years old, if the surgery is very bleeding or if the creatinine clearance is < 50 mL/min.
In Europe, apixaban is accepted for the following indications:
- Prophylaxis of postoperative deep vein thrombosis (2.5 mg 2x/d) [20];
- Treatment of acute deep vein thrombosis and pulmonary embolism (10 mg 2x/d for 7 days, then 5.0 mg 2x/d);
- Prevention of systemic embolism in non-valvular AF (5 mg 2x/d) [9].
Edoxaban (Savaysa® , Lixiana® )
Edoxaban has been introduced in the Japanese, US and European markets since spring 2015 for the treatment and prevention of DVT and for stroke prevention in AF [7,8]. At a dose of 60 mg/d, it significantly reduces the risk of bleeding compared to warfarin [17]. It has a half-life of 10-14 hours and half of its elimination is renal. Its dosage is 30-60 mg once a day (independently of meals), but it should be halved in cases of renal insufficiency (creatinine clearance 30-50 mL/min) [38]. Like all xabans, it prolongs PT non-quantitatively, but only partially modifies TT and PTT; the test of choice is the anti-Xa activity assay [11]. Since December 2016, there is a specific test for edoxaban (STA™-Edoxaban Control, Stago Inc) allowing a quantitative determination. As there are no data on its management perioperatively, it is currently suggested to stop it 48 hours before surgery in standard cases, 72 hours before spinal LRA and 3-4 days in case of renal failure (Tables 8.7 8.11 and 8.12 ). Prothrombin complex 4-factor concentrates (50 IU/kg) antagonise the effect of edoxaban [39].
Contraindications
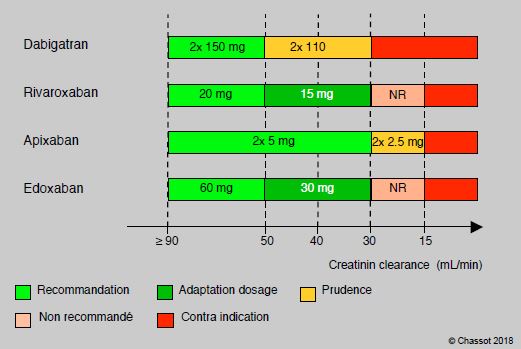
Generally speaking, the various NOACs (new oral anticoagulants: dabigatran, rivaroxaban, apixaban, edoxaban) share the same contraindications. These are mainly related to the risk of bleeding and the renal elimination (total or partial) of these substances (Figure 8.11B).
- Clinically significant progressive bleeding, bleeding diathesis, coagulopathy;
- Ulcerative gastrointestinal disease, severe liver disease;
- Renal insufficiency (creatinine clearance < 30 mL/min or requiring dialysis);
- Pregnancy and breastfeeding;
- Heart valve prosthesis (indication for AVK).
Figure 8.11B: Dosage adjustment and contraindication of NOACs according to creatinine clearance. The proportion of renal elimination varies between substances: dabigatran > 80%, edoxaban 45%, rivaroxaban 35%, apixaban 25% [from 13].
Antidote
Unlike dabigatran, anti-Xa agents do not have an antagonist available for emergency use. However, two agents are currently in clinical trials and are expected to reach the market in the near future (see Antagonism) [4,22,29].
- Andexanet alfa (Andexxa® ); works as a decoy for factor Xa and sequesters factor Xa inhibitors (xabans). Anti-Xa activity is reduced by 92-94% in 5 minutes and the effect lasts for 2 hours [32] and haemostasis is effective in 79% of cases [10]. As the duration of action of andexanet is shorter than that of xabans, there is a risk of rebound anticoagulation. The substance is being marketed to reverse the effect of rivaroxaban, apixaban and edoxaban; it was approved by the FDA in April 2018, and soon by the EMA.
- Ciraparantag (aripazine, PER977); a versatile molecule containing 8 covalent H+-binding sites , effective in reversing the effect of xabans, argatroban, fondaparinux, dabigatran and heparins. Clotting time is normalised within 10 minutes [2], and bleeding is reduced by 90% in animals [21]. As clinical trials are not yet complete, the substance will not be on the market before 1-2 years.
| Anti-Xa agents |
|
Direct factor Xa inhibitors, administered orally once daily (2x for apixaban). The specific test is the calibrated anti-Xa activity for the drug, as the usual tests are non-quantitatively modified; only the PT is prolonged. Drug interference is possible with substances whose metabolism is also dependent on the liver cytochromes CYP3A4 and the transport protein P-gp.
- Rivaroxaban (Xarelto® , 10-20 mg/d), half-life: 10 hours, 12 hours > 75 years and if renal insufficiency. Preoperative withdrawal time: minimum 24 hours (5-10 mg/d) before minor surgery; 48 hours before major surgery, dosage 15-20 mg/d, or age >75 years; 2-4 days in case of renal failure (renal elimination for half)
- Apixaban (Eliquis® , 2.5-5.0 mg 2x/d), half-life 12 hours, elimination mainly by hepatic metabolism. Preoperative withdrawal time: 48 hours
- Edoxaban (Lixiana® 60 mg/d), half-life 10-14 hours, half eliminated by kidney. Preoperative withdrawal time: 48 hours, 72 hours if there is a risk of bleeding or spinal LRA
- As a general rule: delay 3 half-lives before surgery (48 h) and 5 half-lives before spinal LRA (72 h)
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- AGENO WA, GALLUS AS, WITTKOWSKY A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e44S-e88S
- ALBALADEJO P, BONHOMME F, BLAIS N, et al. Management of direct oral anticoagulants in patients undergoing elective surgeries and invasive procedures: update guidelines from the French Working Group on Perioperative Hemostasis. Anaesth Crit Care Pain Med 2017; 36:73-6
- ANSEL JE, BAKHRU SH, LAULICHT BE, et al. Use of PER977 to reverse the antocoagulant effect of edoxaban. N Engl J Med 2014; 371:2141-2
- ARBIT B, NISHIMURA M, HSU JC. Reversal agents for direct oral anticoagulants: a focused review. Int J Cardiol 2016; 223:244-50
- BAUER KA. Recent progress in anticoagulant therapy: oral direct inhibitors of thrombin and factor Xa. J Thromb Haemost 2011; 9(suppl 1):12-19
- BAUERSACHS R, BERKOWITZ SD, BRENNER B, et al, EINSTEIN investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499-510
- BOUNAMEAUX H, CAMM AJ. Edoxaban: An update on the new oral direct factor Xa inhibitor. Drugs 2014; 74:1209-31
- CAMM AJ, BOUNAMEAUX H. Edoxaban. A new oral direct factor Xa inhibitor. Drugs 2011; 71:1503-26
- CONNOLLY SJ, EIKELBOOM J, JOYNER C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806-17
- CONOLL SJ, MILLING TJ, EIKELBOOM JW, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med 2016; 375:1131-41
- CUKER A, HUSSEINZADEH H. Laboratory measurement of the anticoagulant activity of edoxaban: a systematic review. J Thromb Thrombolysis 2015; 39:288-94
- CUKER A, SIEGAL DM, CROWTHER MA, et al. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 2014; 64:1128-39
- EHRA - European Heart Rhythm Association. EHRA practical guide on the use of non-vitamin K antagonists and oral anticoagulants in patients with atrial fibrillation. www.escardio.org/EHRA, 2018
- EIKELBOOM JW, CONNOLLY SJ, BOSCH J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377:1319-30
- GALANIS T, THOMSON L, PALLADINO M, et al. New oral anticoagulants. J Thromb Thrombolysis 2011; 31:310-20
- GIBSON CM, MEHRAN R, BODE C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016; 375:2423-34
- GIUGLIANO RP, RUFF CT, BRAUNWALD E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369:2093-104
- HEIDBUCHEL H, VERHAMME P, ALINGS M, et al. European Heart Rythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015; 17:1467-507
- HEIDBUCHEL H, VERHAMME P, ALINGS M, et al. Updated European Heart Rythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Executive summary. Eur Heart J 2017; 38:2137-49
- LASSEN MR, GALLUS A, RASKOB GE, et al, ADVANCE-3 investigators. Apixaban versus enoxaparin for thromboprophylaxis aTFer hip replacement. N Engl J Med 2010; 363:2487-98
- LAULICHT B, BAKHRU S, JIANG X. Antidote for new oral anticoagulants: mechanism of action and binding specificity of PER977. J Thromb Haemost 2013; 11:75
- LU G, DEGUZMMAN FR, HOLLENBACH SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446-53
- MAR PL, FAMILTSEV D, EZEBOWITZ MD, et al. Periprocedural management of anticoagulation in patients taking novel oral anticoagulants: Review of the literature and recommendations for specific populations and procedures. Int J Cardiol 2016; 202:578-85
- MEGA JL, BRAUNWALD E, MOHANAVELU S, et al. Rivaroxaban versus placebo in patients with acute coronary syndrome (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 2009; 374:29-38
- MEGA JL, BRAUNWALD E, WIWIOTT SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9-19
- MUECK W, LENSING AWA, AGNELLI G, et al. Rivaroxaban. Population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet 2011; 50:675-86
- MUECK W, STAMPFUSS J, KUBITZA D, BECKA M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 2014; 53:1-16
- PATEL MR, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883-81
- RUFF CT, GIUGLIANO RP, ANTMAN EM. Management of bleeding with non-vitamin K antagonist oral anticoagulants in the era of specific reversal agents. Circulation 2016; 134:248-61
- SAMAMA MM, CONTANT G, SPIRO TE, et al. Laboratory assessment of rivaroxaban: a review. Thrombosis Journal 2013; 11:11
- SIÉ P, SAMAMA CM, GODIER A, et al. Surgeries and invasive procedures in patients treated long-term with an oral anti-IIa or anti-Xa direct anticoagulant. Proposals from the Perioperative Haemostasis Interest Group (GIHP) and the Haemostasis and Thrombosis Study Group (GEHT). Ann Fr Anesth Réanim 2011; 30: 645-50
- SIEGAL DM, CURNUTTE JT, CONNOLLY SJ, et al. Andexanet alfa for reversal of factor Xa inhibitor activity. N Engl J Med 2015; 373:2413-24
- SINAURIDZE EI, PANTELEEV MA, ATAULLAKHANOV FI. Anticoagulant therapy: basic principles, classic approaches and recent developments. Blood Coag Fibrinol 2012; 23:482-93
- SPAHN DR, BEER JH, BORGEAT A, CHASSOT PG, et al. New oral anticoagulants in anesthesiology. Transf Med Hemother 2019; 46:282-93
- SPYROPOULOS AC, AGENO W, ALBERS GW, et al. Rivaroxaban for thromboprophylaxis aTFer hospitalization for medical illness. N Engl J Med 2018; 379:1118-27
- TURPIE AG, KREUTZ R, LLAU J, et al. Management consensus guidance for the use of rivaroxaban - an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876-86
- TURPIE AG, LASSEN MR, DAVIDSON BL, et al, RECORD4 investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis aTFer total knee arthroplasty: a randomised trial. Lancet 2009; 373:1673-80
- WEITZ JI, EIKELBOOM JW, SAMAMA MM. New antithrombotic drugs. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e120S-e151S
- ZAHIR H, BROWN KS, VANDELL AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by 4-factor prothrombin complex concentrate. Circulation 2015; 131:82-90