From the start of the ECC, haemodilution increases the volume of distribution (Vd) of substances in solution in the central compartment and abruptly decreases their concentration. This leads to two phenomena (Figure 7.28) [2].
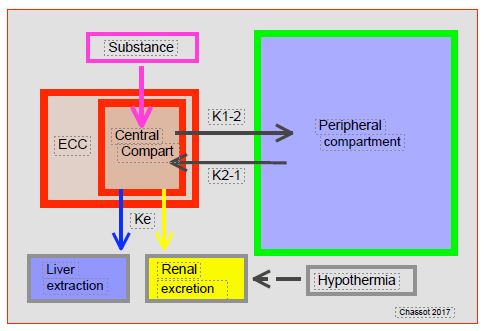
Figure 7.28: Pharmacokinetic effect of ECC. The volume provided by the ECC increases the central compartment by 20-35%, where the substances are administered and from which hepatic extraction and renal filtration take place (depending on the elimination constant Ke). This compartment is in equilibrium with the rest of the body (peripheral compartment) according to concentration gradients. Substances diffuse from the centre to the periphery (K1 → 2) during the distribution phase and from the periphery to the centre (K2 → 1) during the elimination phase. Hypothermia slows down hepatic extraction and renal elimination.
- As fat-soluble substances, anaesthetic agents are less soluble in crystalloids than in whole blood; their circulating concentration decreases with increase in priming fluid [4].
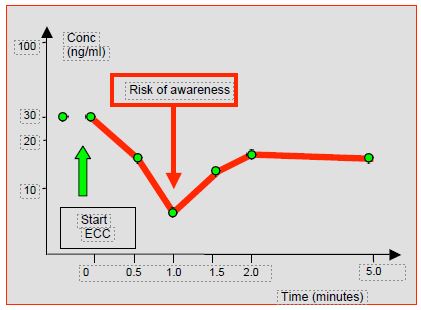
- The sudden decrease in the free concentration of substances, which is responsible for the clinical effects and controls diffusion/elimination, involves a risk of awakening and decurarisation [8,13]. Concentration variations may differ up to10-35% depending on the substances and their volumes of distribution (Figure 7.29) [6,19].
Figure 7.29: Plasma concentration profile of fentanyl at the start of ECC. The fall in level is brief; balance is restored within 2 minutes [13].
Furthermore, the decrease in the concentration of albumin and acidic alpha-glycoprotein, which transport acidic and alkaline substances respectively, is 30-40%. The clinical effect will depend on the extent of protein binding. The free rate of highly bound substances (e.g. propofol 98%, midazolam 94%, fentanyl 84%) increases significantly, while that of weakly bound substances (e.g. vecuronium 30%, morphine 35%) changes marginally [19]. In addition, there is the effect of heparin. In high concentrations (≥ 300 IU/kg), heparin induces a release of lipoprotein lipase and hepatic lipase, which hydrolyses plasma triglycerides into free fatty acids (FFA), which increase by 15-20% [10]. These free fatty acids bind to plasma proteins and displace substances bound to them, thus increasing their free concentration. This process takes place within minutes [19]. The clinical effect of propofol decreases very momentarily due to the increase in its volume of distribution at the beginning of the ECC, but the increase in its free fraction tends to restore its normal hypnogenic activity [3,4]. With high-dose infusion, its activity tends to be more pronounced (lower BIS) [23]. Dexmedetomidine is still poorly studied, but there is some evidence that its clearance decreases in ECC [21].
There are therefore two groups of effects that can partially offset each other, but which do not have the same kinetics and are transient. They last for the time necessary for redistribution from the tissues, which will rebalance the concentration gradients (K2 diffusion® 1 in Figure 7.29) [11]. Indeed, the volume of distribution (Vd) of anaesthetic agents, which are lipophilic substances that penetrate cells, is much larger than the volume of the ECC, and acts as a reservoir for them. For curares, which are hydrophilic substances that remain outside the cells, the Vd is smaller, the protein binding weaker, and the sudden dilution more important. These changes in concentration last only a few minutes and depend on the ratio of the volume of the bypass machine to the patient's circulating volume; the smaller the patient's circulating volume, the greater the change [6]. In practice, this means that children may wake up and/or decurarise suddenly during the first minutes of ECC if the concentration of the substance is not maintained by addition to the bypass priming fluid or to the patient. Adults may decurarise.
Hypothermia slows down digestive, subcutaneous and intramuscular resorption, slows down the distribution of substances between the different compartments, inhibits their biotransformation (decrease in the Hoffman reaction), and reduces their clearance by liver and kidneys. For example, inhibition of hepatic rhodanases delays the metabolisation of nitroprusside. Receptor affinity is decreased as temperature drops. Thus the effect of curares is increased during hypothermic bypass surgery at 28°C [7,9]. Cold-induced vasoconstriction reduces the volume of distribution of substances with a low Vd; this is the case for curares, which increase in hypothermic bypass surgery. In addition, hypothermia gradually increases intracellular water and decreases plasma volume [24]. The elimination half-life of fentanyl is extended by 25% through hypothermia and ECC. The elimination half-life of alfentanil is almost tripled: from 72 minutes prior to bypass surgery to 195 minutes after restarting the pump [12]; this means a reduction in the infusion rate of alfentanil from 3 mcg/kg/min prior to bypass surgery to 1 mcg/kg/min after bypass. In addition, the decrease in neural activity associated with temperature drop decreases the need for anaesthetic and analgesic agents to maintain sleep and pain relief; similarly, curare doses are lower in hypothermia [4].
As gas solubility increases at low temperatures, hypothermia increases the dilution of halogens in the blood and decreases their tissue partial pressure, so the concentration must be increased to achieve the same effect (increase in apparent MAC): below 30°C, the blood concentration of isoflurane is half the inspired fraction displayed on the vaporiser [20]. However, the need for anaesthetic agents is reduced by hypothermia, as there is no conscious activity below a brain temperature of 30-32°. The effective MAC of halogen decreases linearly between 37° and 20°, where it becomes zero [1]. These two effects therefore tend to offset each other.
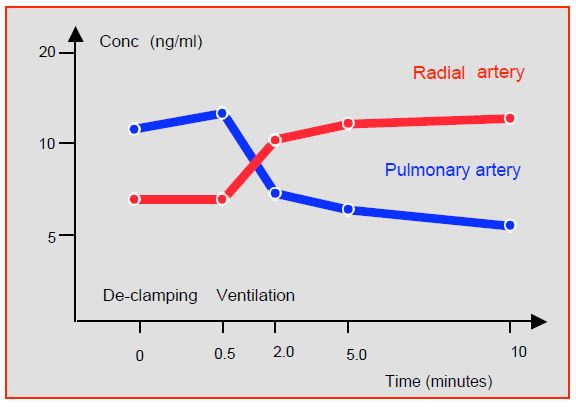
The lungs act as a reservoir for basic fat-soluble substances such as fentanyl, sufentanil, propofol or lidocaine, which remain stored there during bypass surgery as there is no pulmonary circulation. They will be released at the time of decompression and reventilation, causing an increase in plasma levels and a momentary deepening of anaesthesia (Figure 7.30) [5,17,22].
Figure 7.30: Changes in plasma fentanyl levels during pulmonary recirculation. The release results in increased radial artery fentanyl levels while venous (pulmonary artery) levels are low [5].
Uptake and metabolism of dopamine and noradrenaline by the lungs stop when the lungs are excluded; if an infusion is in progress, serum levels increase during the clamp period.
Hepatic and renal flow decreases in bypass surgery due to hypothermia and depulsation. This has a significant effect on the metabolism of substances that have a high (fentanyl, vecuronium) or medium (alfentanil) hepatic extraction coefficient and on those that are excreted by the kidneys [15]. Lactate clearance is decreased by 30% during ECC [16]. However, these phenomena are only clinically significant at temperatures < 28°C [4]. On the other hand, flow decreases due to surgical contingencies, hypothermia, peripheral vasoconstriction and depulsation lead to hypoperfusion, which causes many cells to become hypoxic and acidotic; upon reperfusion and rewarming, basic substances that have accumulated in acidic tissues will be released into the circulation. Hypotension, vasoconstriction and depulsation of arterial flow also decrease peripheral flow in muscles and organs; substances are sequestered in these tissues during low flow and released on rewarming. The kidneys interpret arterial depulsation as hypotension and trigger the renin-angiotensin system.
The components of ECC systems, particularly oxygenators, absorb some lipophilic substances such as fentanils, midazolam, propofol or halogens [14]. However, this effect is limited in scope due to the large volume of distribution of these substances and the re-equilibration between the compartments. This effect is difficult to quantify as the materials used are constantly evolving and reliable data are rarely available. Haemofiltration, on the other hand, is effective in removing a large number of substances from the central compartment of the circulation, since it extracts molecules up to 20,000 Dalton.
The systemic inflammatory response and the release of endotoxins inhibit cytochrome P450 function and reduce the metabolism of many substances; this effect is prolonged beyond the time of ECC. The brain cytochrome P450 is similarly inhibited, resulting in a slowing of the local metabolism of drugs with neuroleptic and hypnogenic effects [18].
All these changes have relatively minor impact in the usual situations, but become important in low weight people (< 50 kg, children), in the elderly (decreased cell mass and plasma protein levels), and in debilitated patients (chronic heart failure, cachexia).
| Pharmacokinetics in ECC |
|
Because of haemodilution and temperature variations, ECC has a major influence on the pharmacokinetics of anaesthetic agents. These effects are variable depending on the substance and temperature; the clinical translation is the result of the various changes.
↓ sudden concentration at the beginning of the ECC (risk of awakening and decurarisation)
↑volume of distribution
↓concentration by sequestration (lungs, ECC circuits)
↑effective concentration and ↑ duration of action by: ↓ clearance, ↓ biotransformation
↓ esterase activity, ↓ receptor affinity
↑of the solubility of (halogenated) gases in hypothermia, thus ↓ of their partial pressure
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- ANTOGNINI J. Hypothermia eliminates isoflurane requirements at 20 degrees celsius. Anesthesiology 1993; 78:1152-7
- AUFFRAY JP, ROCH A. Medication and extracorporeal circulation. In: JANVIER G, LEHOT JJ. Circulation extracorporelle: principes et pratique. Rueil-Malmaison: Arnette 2004, 387-8
- BAILEY JM, MORA CT, SHAFER SL, et al. Pharmacokinetics of propofol in adult patients undergoing coronary revascularization. Anesthesiology 1996; 84:1288-97
- BARRY AE, CHANEY MA, LONDON MJ. Anaesthetic management during cardiopulmonary bypass: a systematic review. Anesth Analg 2015; 120:749-69
- BENTLEY JB, CONAHAN TJ, CORK RC. Fentanyl sequestration in lungs during cardiopulmonary bypass. Clin Pharmacol Ther 1983;34:703-6
- BUYLAERT W, HERREGODS L, MORTIER L, et al. Cardiopulmonary bypass and the pharmacokinetics of drugs. Clin Pharmacokinet 1989; 17:10-5
- BUZELLO W, SCHLUERMANN D, POLLMAECHER T, et al. Unequal effects of cardiopulmonary bypass-induced hypothermia on neuromuscular blockade from constant infusion of alcuronium, d-tubocurarine, pancuronium, and vecuronium. Anesthesiology 1987; 66:842-6
- DAWSON PJ, BJORKSTEN AR, BLAKE DW, et al. The effects of cardiopulmonary bypass on total and unbound plasma concentration of propofol and midazolam. J Cardiothorac Vasc Anesth 1997; 11:556-61
- DIEFENBACH C, ABEL M, BUZELLO W. Greater neuromuscular blocking potency of atracurium during hypothermic than during normothermic cardiopulmonary bypass. Anesth Analg 1992; 75:675-8
- HAMMAREN E, YLI-HANKALA A, ROSENBERG PH, et al. Cardiopulmonary bypass-induced changes in plasma concentration of propofol and in auditory evoked potentials. Br J Anaesth 1996; 77:360-4
- HUDSON RJ, THOMPSON IR, JASSAL R, et al. Cardiopulmonary bypass has minimal effect on the 142 - pharmacokinetics of fentanyl in adults. Anesthesiology 2003; 99:847-54
- HUG CC, DELANGE S, BURM AGL. Alfentanil pharmacokinetics in patients before and after cardiopulmonary bypass. Anesth Analg 1983; 62:245
- HYNYNEN M. Binding of fentanyl and alfentanil to the extracorporeal circuit. Acta Anaesthesiol Scand 1987; 31:706-10
- HYNYNEN M, HAMMAREN E, ROSENBERG PH. Propofol sequestration within the extracorporeal circuit. Can J Anaesth 1994; 41:583-8
- McKINDLEY DS, HANES S, BOUCHER BA. Hepatic drug metabolism in critical illness. Pharmacotherapy 1998; 18:759-78
- MUSTAFA I, ROTH H, HANAFIAH A, et al. Effect of cardiopulmonary bypass on lactate metabolism. Intensive Care Med 2003; 29:1279-85
- OKUTANI R, PHILBIN D, ROSOW C. Effect of hypothermic hemodilutional cardiopulmonary bypass on plasma sufentanil and catecholamine concentrations in humans. Anesth Analg 1988; 67:667-73
- RENTON KW, NICHOLSON TE. Hepatic and central nervous system cytochrome P450 are down-regulated during lipopolysaccharide-evoked localized inflammation in brain. J Pharmacol Exp Ther 2000; 294:524-30
- ROSEN D, ROSEN K. Elimination of drugs and toxins during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997; 11:337-40
- SMITH D. Cardiovascular and pulmonary pharmacology. In: ALSTON RP, MYLES P, RANUCCI M, eds. Oxford Textbook of Cardiothoracic Anesthesia. Oxford: Oxford University Press, 2015, 69-82
- SU F, GASTONGUAY MR, NICOLSON SC, et al. Dexmedetomidine pharmacology in neonates and infants after open heart surgery. Anesth Analg 2016; 122:1556-66
- TAEGER K, WENINGER E, SCHMELZER F, et al. Pulmonary kinetics of fentanyl and alfentanil in surgical patients. Br J Anaesth 1988; 61:425-34
- TAKIZAWA E, HIRAOKA H, TAKIZAWA D, et al. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br J Anaesth 2006; 96:179-85
- WONG KC. Physiology and pharmacology of hypothermia. West J Med 1983; 138:227-32