Despite half a century of research into cardioplegia, the ideal solution has still not been found. Therefore, there are many variations in these infusates.
- Hyperkalemic and hypothermic crystalloid solution (5-10°);
- Blood with potassium solution;
- Cardioplegia with hypothermic or normothermic blood;
- Intermittent or continuous infusion;
- Addition of metabolic substrate: glucose, amino acids, ATP;
- Addition of protective agents.
The aim of cardioplegia is to fulfil six physiological requirements: immediate cardiac arrest, metabolic arrest, substrate supply, pH maintenance, prevention of oedema, and prevention of reperfusion injury [3].
Crystalloid solutions
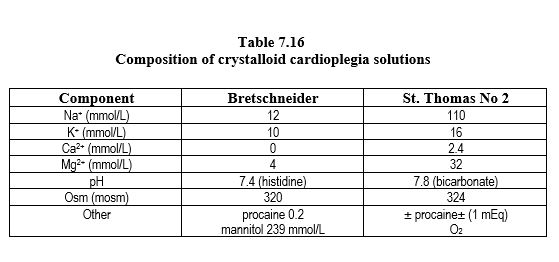
Crystalloid cardioplegia solution is the simplest and least expensive solution and is effective in standard cases. The most commonly used solutions are extracellular solutions enriched in potassium and low in calcium, such as St. Thomas' solution (Table 7.16) [5]. Other solutions mimic the intracellular composition (low in sodium and free of calcium), such as Bretschneider's solution or HKT Custodiol™ solution; they are also used for organ preservation in transplantation. Magnesium is usually added to decrease the increase in [Ca2+ ]i caused by hyperkalaemia. The advantage of crystalloid cardioplegia is the clarity of the surgical field for the surgeon and the favourable rheology for reaching areas distal to tight coronary obstructions, but the disadvantage is the inability to transport oxygen and the risk of haemodilution [2]. It is increasingly being replaced by blood cardioplegia.
The solutions are administered by a separate pump from the bypass circuit (Figure 7.13) at a pressure of approximately 70-150 mmHg and a flow rate of 200-300 mL/min over at least 2 minutes, as 2 mL of solution is required per gram of heart, i.e. approximately 500 mL for an adult. For maintenance of arrest, it is sufficient to repeat the infusion every 30 minutes at a rate of 150 mL/min over 1-2 minutes. The temperature is 5-10°. By retrograde route, the infusion pressure is about 20-30 mmHg, with a maximum value of 40 mmHg.
One modification of crystalloid cardioplegia is to administer a small volume of a highly concentrated solution [2]. These very high concentrations, especially of K+ , are possible because the amount infused is approximately the volume of the coronary tree, so that most of the solution remains in the heart and does not enter the general circulation. This small volume does not allow for significant heat exchange; protection, lasting 30-60 minutes, is provided by the composition of the solution but not by hypothermia.
- Custodiol™, a hyperkalaemic solution low in Na+ and Ca2+ but with added histidine, keto-glutarate and mannitol, given as a single dose of 5 mL/kg over 7-10 minutes.
- Cardioplexol™, a hyperkalaemic (100 mmol/L) and hypermagnesaemic (162 mmol/L) solution with added xylitol, citric acid and procaine; given as a 100 mL bolus, which represents 10 mmol K+ , 16.2 mmol Mg2+ and 300 mg procaine; protects the heart for 45 minutes, regardless of temperature
Blood cardioplegia
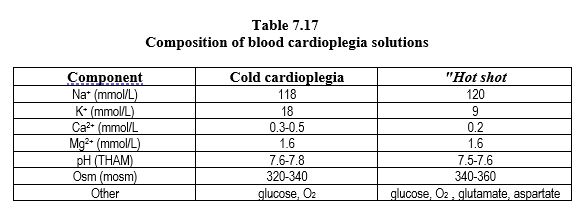
Arterial blood drawn from the bypass circuit by a separate pump is supplemented with a potassium-rich electrolyte solution in a 1:5 solution to blood ratio. It passes through an independent heat exchanger (Figure 7.13). It is an alkaline (pH ≥ 7.6) and hyperosmolar (≥ 330 mOsm) perfusate, containing 20-25 mmol/L potassium and little calcium (≤ 1 mmol/L) (Table 7.17). This takes advantage of the oxygen-carrying capacity of blood, its buffering capacity, and its rheological, oncotic and free radical antagonistic qualities. The histidine in plasma proteins provides most of the buffering capacity, which is effective at any temperatures. Hypothermic blood cardioplegia is currently the most frequently used cardioplegia technique [8].
Compared to crystalloid solution, blood cardioplegia gives better results, especially in high-risk cases: better functional recovery, less ischaemic damage, more frequent recovery of spontaneous sinus rhythm, better preservation of ATP reserves [6]. However, there is no reduction in the rate of infarction or mortality [8].
A variation in the solution/blood ratio consists of injecting a concentrated cardioplegic solution containing 16 mmol/L KCl and 3 mmol/L Mg2+ into a flow of blood taken from the arterial line of the bypass graft, whose temperature is > 30°C, via a syringe pump (at 45 mL/h). This so-called Calafiore technique limits the degree of haemodilution [4].
Blood cardioplegia can be administered at three different temperatures [1,6,7].
- Arrest is usually induced by a cold infusion (5-10°C) of 2-4 minutes at 200-300 mL/min and a pressure of 60-150 mmHg; mechanical stillness is maintained by iterative infusions at 150 mL/min. Low temperatures improve protection and allow time off between infusions (30-40 minutes), but reperfusion injury is still very important.
- Normothermic blood cardioplegia infusion can be used exclusively, but should be continuous or repeated every 10 minutes. If the intramyocardial distribution is inadequate (hypertrophic heart disease, severe coronary artery disease), then the heart is at risk of ischaemic damage because the metabolism is not slowed down. Compared to cold cardioplegia, "warm" cardioplegia tends to improve postoperative ventricular function and decrease ischaemic markers (troponins, CK-MB), mainly in cases where it can remain continuous throughout the procedure.
- A compromise is sought in a warm intermediate temperature (27-30°C), which has been shown to be effective for myocardial protection and attenuates reperfusion injury.
- Prior to revascularisation, normothermic (hot shot) cardioplegia infusion for 2-3 minutes at 60 mmHg reduces reperfusion injury.
Added substances
Cardioplegia solutions should be slightly hyperosmolar to reduce oedema, slightly alkaline to reduce lactic acidosis, and contain little calcium to minimise intramitochondrial calcium accumulation. Many additives are used to fulfil these functions.
- Buffer: Tris, THAM, histidine (bicarbonate loses its buffer effect when cold);
- Hyperosomolar agents (mannitol, albumin, colloid);
- Magnesium, steroid, procaine;
- ATP, glucose;
- Anti-oxidant agents.
It should be remembered that preconditioning with halogenated agents (isoflurane, sevoflurane and desflurane at 1-2 MAC) during the operation improves myocardial tolerance to ischaemia.
| Cardioplegia techniques |
|
Cardioplegia techniques can be divided into two groups:
- Hyperkalemic and hypothermic crystalloid solutions
- Cardioplegia with blood, with addition of K+ ; advantages: supply of O2 and ATP, buffer (valance histidine)
Arrest is caused by hyperkalaemia, usually cold (5-10°C). Normothermic blood solution reduces reperfusion injury but must be injected without interruption.
Many substances can be added depending on the protocols.
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- ABAH U, ROBERTS PG, ISHAQ M, et al. Is cold or warm cardioplegia superior for myocardial protection? Interact CardioVasc Thorac Surg 2012; 14 :848-55
- ANGELI E. The crystalloid cardioplegia: advantages with a word of caution. Ann Fr Anesth Réan 2011; 30:S17-S19
- BUCKBERG GD. Strategies and logic of cardioplegic delivery to prevent, avoid and reverse ischemic and reperfusion damage. J Thorac Cardiovasc Surg 1987; 93:127-35
- CALAFIORE AM, TEODORI G, MEZZETTI A, et al. Intermittent antegrade warm blood caredioplegia. Ann Thorac Surg 1995; 59:398-402
- CHAMBERS DJ, SAKAI A, BRAIMBRIDGE MV, et al. Clinical validation of St. Thomas's Hospital cardioplegic solution No. 2 (Plegisol). Eur J Cardiothorac Surg 1989; 3:346-52
- DECOENE C. Blood cardioplegia: advantages and disadvantages. Ann Fr Anesth Réan 2011; 30:S20-S22
- FAN Y, ZHANG AM, XIAO YB, et al. Warm versus cold cardioplegia for heart surgery: A meta-analysis. Eur J Cardiothorac Surg 2010; 37:912-9
- JACOB S, KALLIKOURDIS A, SELKE F, DUNNING J. Is blood cardioplegia auperior to crystalloid cardioplegia? Interact CardiioVasc Thor Surg 2008; 7:491-9