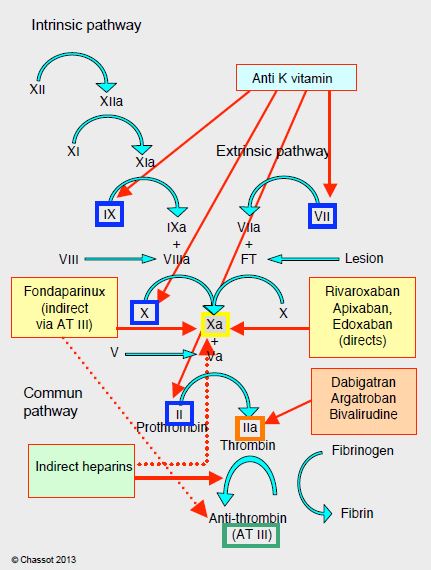
Anticoagulants currently in clinical use can be classified into four categories according to their sites of action (Figure 8.9).
Figure 8.9: Impact points of different anticoagulants on the coagulation cascade: anti-vitamin K agents (blue), anti-Xa agents (yellow), direct anti-thrombin agents (orange), heparins (green) (AT III mediated anti-thrombin agents). Low molecular weight heparins have a strong anti-Xa effect (dotted arrow). AT: antithrombin.
- Indirect thrombin inhibitors: heparins, pentasaccharides (fondaparinux) (intravenous or subcutaneous);
- Direct thrombin inhibitors: intravenous (lepirudin, bivalirudin, argatroban) or oral (dabigatran);
- Direct factor Xa inhibitors: rivaroxaban, apixaban, edoxaban (oral);
- Anti-vitamin K agents: warfarin, coumarins (per os).
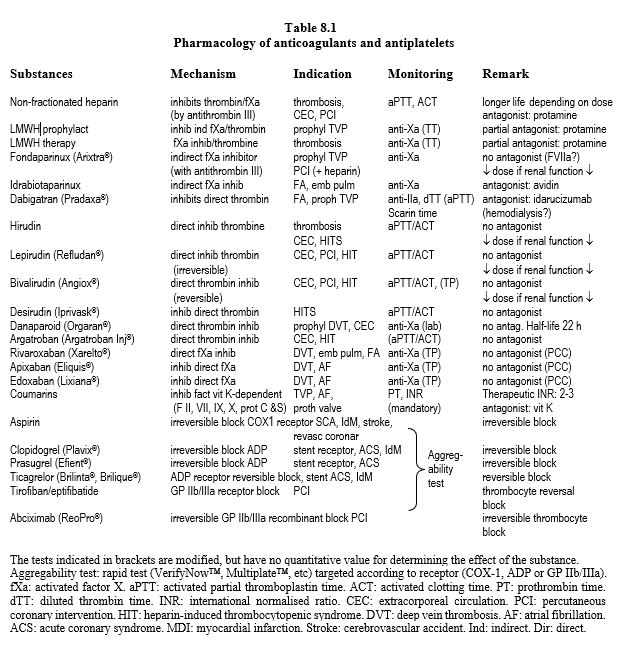
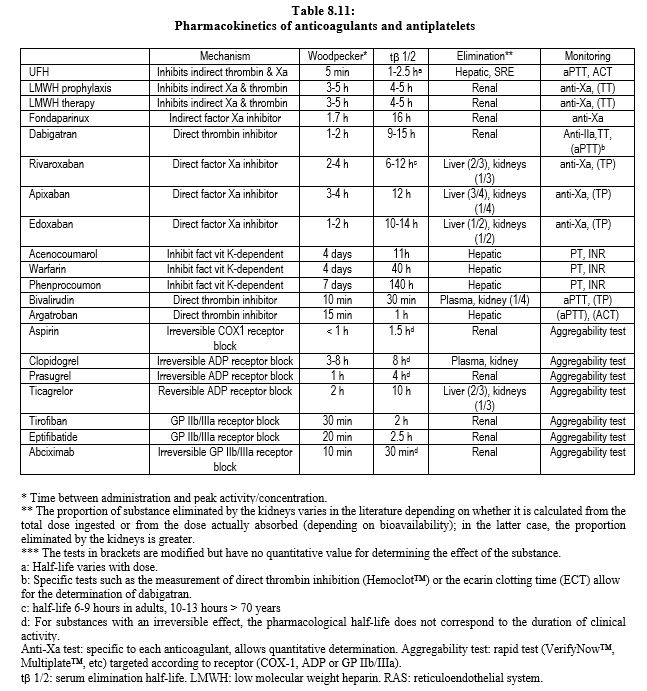
Conventional anticoagulants, which have been in clinical use for a long time, have several points of impact in the coagulation chain, whereas the new substances (NOAC: new oral anticoagulants) are much more selective. On the other hand, the latter have quick onset, have fewer interactions, and have more predictable pharmacokinetics that make monitoring of the anticoagulant effect unnecessary. Overall, NOACs are more effective than AVKs in thromboembolic prevention (mean RR 0.81), have a much lower risk of intracranial haemorrhage (RR 0.48), and offer a small reduction in mortality (RR 0.90) [9]. They are indicated for thromboembolism and non-valvular AF. This term excludes AF occurring in the context of rheumatic mitral stenosis, mitral plasty with prosthetic material, and mechanical valve replacement or bioprosthesis; these four situations remain in the domain of AVKs [1,4]. AVKs demonstrate a clear superiority compared to NOACs in preventing thromboembolic complications in mechanical heart valves [2]. On the other hand, NOACs can be used in native valve disease such as aortic stenosis, mitral insufficiency or aortic insufficiency. They have a higher risk of gastrointestinal bleeding (RR 1.25), but this type of bleeding causes 10 to 30 times less mortality and disability than a haemorrhagic stroke. Unfortunately, these new drugs have three drawbacks.
- No antagonist (except for dabigatran);
- Variable but non-quantitative effects on standard coagulation tests;
- Renal elimination.
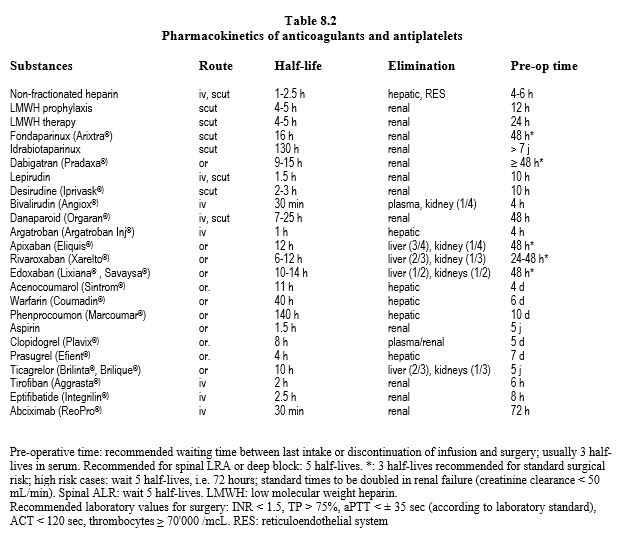
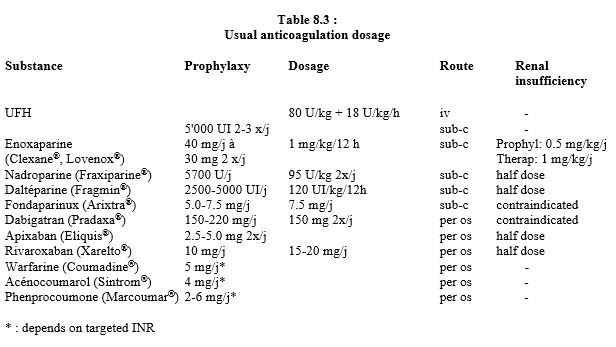
NOACs are largely eliminated by kidneys: completely for fondaparinux and dabigatran, half for edoxaban, one third for rivaroxaban and one quarter for apixaban (see Table 8.11). The elimination half-life is extended depending on the degree of renal impairment [1].
- Creatinine clearance 30-50 mL/min.
- Dabigatran: 18 hours;
- Rivaroxaban, apixaban, edoxaban: 10-15 hours;
- Creatinine clearance 15-29 mL/min.
- Dabigatran: 27 hours;
- Rivaroxaban: 12-15 hours;
- Apixaban: 17 hours;
- Edoxaban: 17 hours;
- Creatinine clearance < 15 mL/min.
- NOACs are in principle contraindicated.
A fourth shortcoming of these substances is their cost: a month's treatment costs an average of €76.00, whereas it costs only €12.50 with AVKs, including the PT test. Although it differs according to the substance, a reduction in dosage is often necessary in the case of hepatic or renal insufficiency, and in case of joint administration of substances modifying anticoagulants metabolism, because risks are cumulative: the addition of several insignificant effects may produce a dangerous result. For renally eliminated substances (LMWH, dabigatran, fondaparinux, xabans), the simplest rule is to give half a dose in case of renal dysfunction (creatinine clearance 30-50 mL/min) and to abstain in case of end-stage failure (creatinine clearance < 30 mL/min). In obese patients, the dose should be increased by 30% [7]. Since data on drug interference are still incomplete, it is prudent to consider the pharmacological contraindications reported for each individual NOAC as valid for all NOACs, i.e. the simultaneous prescription of the following substances [1,4,10].
- Anti-HIV protease drugs (ritonavir, indinavir, atazanavir, etc);
- Azole anti-fungals (ketoconazole, itraconazole, miconazole, voriconazole, etc);
- The immunosuppressants cyclosporine and tacrolimus;
- The antiarrhythmic drugs quinidine, amiodarone and verapamil;
- Rifampicin;
- Carbamazepine, phenobarbital and phenytoin.
Chronic anticoagulant treatment presents major risks, as it is associated with approximately 5,000 deaths per year in France. The combination of anticoagulants with antiplatelet agents carries a prohibitive risk of bleeding, but may be justified in case of acute coronary syndrome or stents in patients with AF or mechanical valve prostheses [5]. This triple therapy involves reducing the doses of xaban, or eliminating aspirin [8]. However, ongoing work suggests a wide field of application for NOACs in cardiovascular disease, where they could compete with antiplatelet agents: as an adjunct in acute coronary syndrome (rivaroxaban 2 x 2.5 mg/d) [6] or in combination with aspirin (rivaroxaban 2 x 2.5 mg/d + aspirin 100 mg/d) in stable coronary artery disease [3], they improve the clinical prognosis.
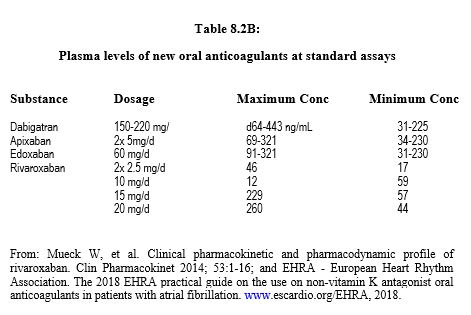
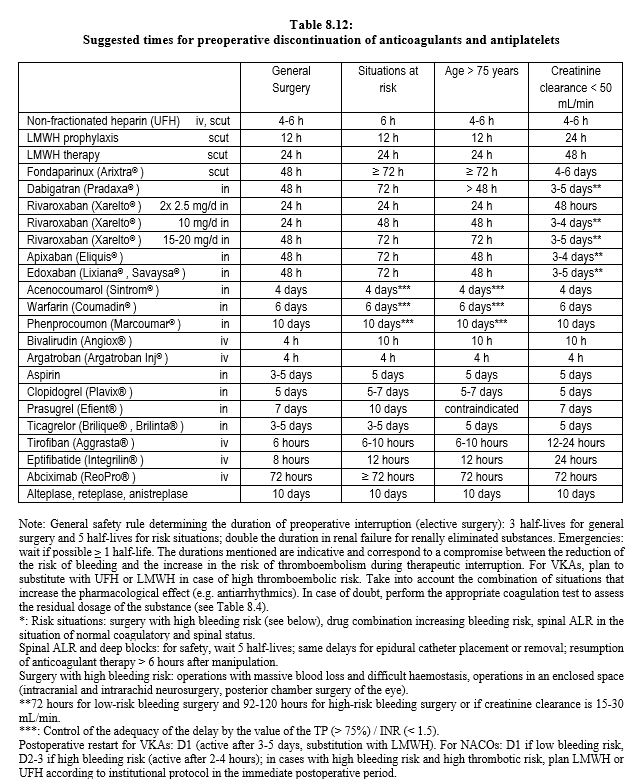
The main data on anticoagulants are summarised in Table 8.1, Table 8.2A and Table 8.2B. Dosages and dose adjustments for renal impairment are given in Table 8.3. Data on antiplatelets are available in Chapter 29 (Chapter 29) and secondarily in Chapter 3 (Antiplatelets). A summary of these data is given in Tables 8.11 and 8.12.
| Anticoagulants |
| Anticoagulants are divided into four groups:
- Indirect thrombin inhibitors (heparins, fondaparinux)
- Direct thrombin inhibitors (bivalirudin, argatroban, dabigatran)
- Direct factor Xa inhibitors (xabans)
- Anti-K vitamin agents (AVK)
The new oral anticoagulants are gradually replacing AVKs, except in cases of heart valve prostheses and rheumatic mitral stenosis. However, they have several shortcomings:
- No antagonism (except for dabigatran)
- Specific complex tests (anti-Xa, anti-IIa)
- Elimination largely renal (prolonged half-life in renal failure)
- Cost
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- DOHERTY JU, GLUCKMAN TJ, HUCKER WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol 2017; 69:871-98
- EIKELBOOM JW, CONNOLLY SJ, BRUECKMANN M, et al. Dabigatran versus warfarin in patients with mechanical heart valves, N Engl J Med 2013; 369:1206-14
- EIKELBOOM JW, CONNOLLY SJ, BOSCH J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377:1319-30
- HEIDBUCHEL H, VERHAMME P, ALINGS M, et al. European Heart Rythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015; 17:1467-507
- HOLBROOK A, SCHULMAN S, WITT DM, et al. Evidence-based management of anticoagulant therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e152S-e184S
- MEGA JL, BRAUNWALD E, WIWIOTT SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012; 366:9-19
- NUTESCU EA, SPINLER SA, WITTKOWSKY A, et al. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother 2009; 43:1064-83
- RAVAL AN, CIGARROA JE, CHUNG MK, et al. Management of patients on non-vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting. Circulation 2017; 135:e604-e633
- RUFF CT, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955-62
- SIÉ P, SAMAMA CM, GODIER A, et al. Surgeries and invasive procedures in patients treated long-term with an oral anti-IIa or anti-Xa direct anticoagulant. Proposals from the Perioperative Haemostasis Interest Group (GIHP) and the Haemostasis and Thrombosis Study Group (GEHT). Ann Fr Anesth Réanim 2011; 30: 645-50