To reduce tissue oxygen consumption, a variable degree of cooling is commun in ECC: mild (32-35°C), moderate (28-31°C) or deep (< 25°C) hypothermia. However, this concept has been challenged over the last twenty years in favour of maintaining the temperature in the normothermic zone (35-37°C). Indeed, advances in bypass surgery technology now allow a high flow rate to be maintained without significant complications, whereas previously it was imperative to lower the flow rate to minimise haematological and tissue damage.
Benefits of hypothermia
Hypothermia affects many systems. Cellular metabolism decreases exponentially with temperature: it drops by 7% per degree centigrade. A cooling of 10° therefore reduces requirements by 50%. The benefits of hypothermia are numerous (Table 7.3).
- Reduction of pump flow (less bleeding in the operating field, less haemolysis); 1.5 L/min/m2 is sufficient to cover the body's needs at 25°C.
- Improved myocardial protection.
- Improved brain protection.
- Reduction of trauma to the blood elements and proteins.
- Reduction of the inflammatory reaction.
- Reduction in the need for allologous blood.
However, hypothermia also has its drawbacks [13].
- Increase in blood viscosity, compensated by a decrease in haematocrit; in hypothermia, viscosity remains approximately stable when Ht in % has the same value as temperature in °C.
- Increased systemic arterial resistance and peripheral vasoconstriction.
- Catecholamine secretion, insulin resistance.
- Functional alterations of proteases of the coagulation cascade.
- Reversible inhibition of platelet adhesion (but preservation of aggregation).
- Leftward shift of the Hb dissociation curve (decreased availability of O2 to the tissues).
- Prolongation of the effect of administered substances (decreased clearances, increased solubility of gases).
- Temperature non-uniformity; organs with high perfusion (brain, kidneys, heart) change their temperature more rapidly than the rest of the body.
- Need to rewarm the patient and risk of hyperthermia, especially the brain.
- Risk of secondary drop of body temperature (afterdrop).
The metabolic rate of decline per 10°C (Q10 ) varies between temperatures, species, organs and ages. In humans, the overall value is 2, i.e. the metabolism decreases by half for every 10°C drop. The decrease in cellular metabolism in cold temperatures allows the duration of ischaemia to be extended by a variable amount depending on the organ. As the brain is the most sensitive structure, the possible ischemia time depends on its own tolerance. At 18°C, the O2 consumption of the brain (CMRO2 ) is 40% of its value in normothermia [20]. Whereas anaesthetic agents only modify electrical activity, hypothermia allows a decrease in the cellular metabolism of neurons (40% of the CMRO2 ) as well as in their synaptic activity (60% of the CMRO2 ) [20,30]. For the brain, the decrease in Q10 between 37° and 27° is due to the reduction in metabolic activity, but the more rapid decrease in CMRO2 between 27° and 18°C (Q10 increased) is attributed to the suppression of electrical activity [21]. The EEG is isoelectric below 20°C. The lower limit of temperature tolerated by the brain is probably 12°C, provided that hypothermia is uniform [19]. Below this value, inhibition of membrane ion pumps allows ions to diffuse along their electrochemical gradients, leading to progressive intracellular oedema [27].
The coupling between blood flow (CBF) and metabolism (normal CBF/CMRO ratio2 : 15/1) changes with cold: at low temperatures, the CBF becomes luxuriant (ratio 30/1). Selfregulation of cerebral blood flow is partially preserved in moderate hypothermia (25-30°C) for mean arterial pressure regimes of 50-80 mmHg and normocapnia, but is lost in deep hypothermia (< 25°C). Selfregulation is most impaired during the rewarming phase and the postoperative stroke rate appears to be directly related to this impairment (odds ratio 6.57) [18]. However, it should be borne in mind that these temperatures may be inhomogeneous; intracerebral gradients may exist, for example between the cortex and the bulb. On the other hand, the location of the measurement is important; the most reliable locations are the jugular bulb (retrograde jugular catheter), the eardrum (foam probe) or the ethmoidal cells (probe inserted through the nose until it reaches the nasopharynx).
Cooling and heating
Systemic cooling through ECC is effective but not uniform. Organs with preferential perfusion (heart, brain, liver, kidneys) cool and warm rapidly, but organs with low blood flow to tissue mass ratios (fat, skin, muscle) change their temperature more slowly. Thermal gradients therefore take place, which can be assessed by measuring two different temperatures.
- Esophageal temperature, which represents the high perfusion organs, but is influenced by a cooled heart ;
- Rectal or bladder temperature, which represents organs with an intermediate blood flow/tissue mass ratio;
- To monitor brain temperature, a probe is placed against the eardrum or against the ethmoidal cells (nasopharyngeal T°); the latter is more reliable before weaning off bypass.
- The T° of the pulmonary catheter is reliable as soon as pulmonary flow is restored.
When cooling or rewarming, thermal gradients of more than 10° between oesophageal and rectal temperatures should be avoided, as this reflects too much inhomogeneity. In addition, dissolved gases change to the gaseous phase when the temperature rises sharply, which happens when warm blood passes through still cold tissue, or vice versa. Gaseous micro-embolisations are then formed, which can lead to capillary occlusions and areas of focal ischaemia.
There are a number of recommendations for temperature management during ECC [8].
- Temperature gradient between water in the heat exchanger and blood should never exceed 10°C, and water temperature should not go above 38°C or below 12°C.
- During cooling, the temperature gradient between the inlet and outlet of the heat exchanger should never exceed 10°C.
- During rewarming when the temperature is < 30°C, the temperature gradient between the inlet and outlet of the heat exchanger must never exceed 10°C.
- During warming up when the temperature is > 30°C:
- The temperature gradient between the inlet and outlet of the heat exchanger must remain ≤ 4°C;
- The heating rate should remain ≤ 0.5°C/min.
- Temperature of blood leaving the heat exchanger should never exceed 37°C to avoid cerebral hyperthermia.
- The gradient between rectal/vesical and oesophageal temperature should remain below 10°C; rectal or bladder T° is 2-4°C below brain temperature during rewarming.
On warming, the brain becomes transiently hyperthermic (38-39°) [4]. Because the brain is one of the best perfused organs and therefore its temperature changes more rapidly than the body during rewarmig. This thermal rebound is more pronounced the faster the warming. It profoundly increases the susceptibility of neurons to ischaemia and increases the extent of focal injury [17,23]. It decreases the efficiency of selfregulation and makes cerebral blood flow more pressure-dependent. Neurological sequelae are proportional to the speed of rewarming [10] and the fall in jugular venous saturation during rewarming [7]. A fall in cerebral O2 saturation (ScO2 ) indicates an imbalance between O2 consumption and brain blood flow. Hyperthermia is responsible for 50-75% of type II neuropsychological changes, the extent of which is directly related to the rate of warming [10,12,14]. This should not exceed 1°C per 5 minutes, nor should the artery-oesophagus temperature gradient exceed 2-3°C [5,17]; however, this progressive rewarming extends the duration of ECC [11]. To avoid cerebral hyperthermia, the most appropriate approach is to focus on four points [11,31]:
- Avoid hypothermia during bypass surgery (T° min > 33°C);
- Heat up slowly (1°C/5 min);
- Maintain the T° of the blood at ≤ 37°C during rewarming (measured on the arterial cannula);
- Wean off from pump at 36° (core temperature).
The last point is highly debatable, as the temperature of the slightly hypothermic patient will drop in the first postoperative hours (afterdrop), since the insufficiently warmed muscle mass represents a cold reservoir that the patient will have to warm up by increasing cardiac output and shivering, conditions associated with a high risk of myocardial ischaemia and a extended extubation time [6]. These risks outweigh the potential benefits. The best compromise is therefore to warm the patient to 36° or 36.5°.
Monitoring brain temperature is challenging because no probe can measure the brain temperature itself, and is also not homogeneous. The three closest measurement points are the jugular venous blood (retrograde catheterisation of the jugular bulb), the eardrum (special foam probe) and the ethmoidal cells (standard nasopharyngeal probe pressed against the posterior-superior wall of the pharynx). Other sites (oesophagus, rectum, bladder, pulmonary artery) may have gradients of up to 5°C relative to brain temperature, and show a significant lag in thermal variation [11,24]. Arterial blood temperature at the heat exchanger outlet correlates best with jugular bulb temperature [8,24].
Temperature control: normothermia versus hypothermia
There is still a lively debate between the two attitudes, "warm" versus "cold" bypass surgery, because both techniques have a particular impact on different body systems [25,29]. Indeed, the benefits of hypothermia are indisputable in complex operations with long aortic clamps, and in circulatory arrest such as in aortic arch surgery. They are much less obvious in simple operations and in low-risk patients. They are also very different for different organs.
- Neuroprotection; hypothermia, even by a few degrees, improves cerebral oxygenation (measured by SvO2 jugular or cerebral saturation ScO2 ) and decreases neurological sequelae [3,31]; it is essential in case of circulatory arrest [32].
- Cardioprotection; cold cardioplegia inhibits myocardial metabolism and provides 30-50 minutes of protection which can be extended by iterative infusions.
- Kidneys and viscera; they do not appear to benefit from hypothermia [33].
- Coagulation; hypothermia alters the activities of coagulation chain proteins and platelet adhesiveness, but these changes are reversible on warming [36].
Normothermia or mild hypothermia (≥ 34°C) provides rather better results than moderate hypothermia (28°) in standard cases. It tends to be used for routine cases.
- Warm cardioplegia offers excellent protection provided that it is continuous or with only brief interruptions (< 12 minutes). Functional recovery of the myocardium after bypass surgery is better and faster than in hypothermia, the rate of spontaneous defibrillation is higher, and the incidence of atrial fibrillation or ventricular assist is halved [1,9].
- The impact on neurological risk is not clearly demonstrated, but there is probably no difference as long as perfusion pressure is properly maintained [25]. Stroke risk and cognitive dysfunction do not appear to differ, probably because the former is primarily embolic in nature and the latter is related to underlying brain pathology [28].
- There is no risk of hyperthermia on rewarming [14].
- The coagulation changes are less and the risk of bleeding is lower [37].
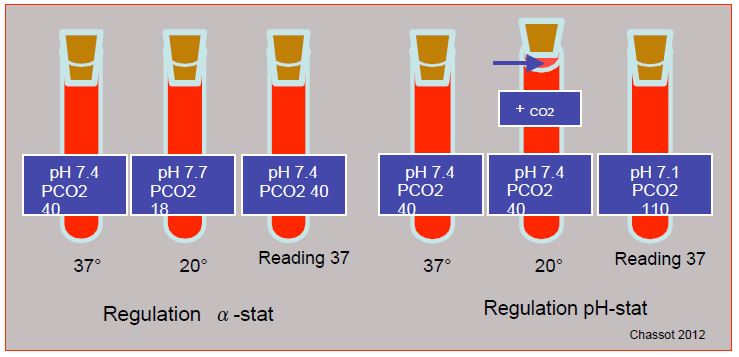
When cold, the solubility of gases in liquids increases. For example, the PCO2 measured in a normal blood sample (PCO2 40 mmHg) cooled to 27°C is only 23 mmHg, even though no exchange with the outside has taken place, because the soluble fraction of the gas [HCO3 ] has increased. At 20°C, the normal apparent pH is 7.7 and the PCO2 is 18 mmHg. In clinical practice, acid-base regulation can be achieved by two techniques (Figure 7.25) [34].
Figure 7.25: Regulation of acid-base balance in hypothermia. In the α-stat mode, the total CO2 content is kept constant, but the reading is taken in a device with the sample reduced to 37°, as if the patient were normothermic. As the solubility of the gas is increased when cold, the partial pressure is actually lower; the blood becomes artificially alkaline and hypocapnic, but the ratio [H+ ]/[OH- ] remains constant. In the pH-stat mode, the pH of the blood is maintained at 7.4 regardless of temperature by adding CO2 to the ventilated gas; the CO content2 increases (apparent hypercarbia) and the pH falls. If the pH is maintained at 7.4 at 17°, the sample read at 37° gives a value of 7.08 (corresponding PCO2 : 110 mmHg).
- In the alpha-stat mode, which is most often used, the total CO2 content is kept constant, but the reading is taken in an apparatus in which the sample is reduced to 37°, as if the patient were normothermic. As the solubility of the gas is increased when cold, the partial pressure is actually lower; the blood becomes artificially alkaline and hypocapnic, but the ratio [H+ ]/[OH- ] remains constant. In this situation, brain selfregulation is intact, extracellular acidosis is reduced, pHi is kept stable, and intracellular enzyme functions are fully preserved [35]. Most cold-blooded animals (poikilotherms) hibernate using this process because glycolysis, which produces little CO2 , is sufficient to meet their low metabolic needs [34].
- In the pH-stat mode, the blood pH is maintained at 7.4 regardless of temperature by adding CO2 to the ventilated gas; the CO content2 increases (apparent hypercarbia) and the pH decreases. If the pH is maintained at 7.4 at 17°, the sample read at 37° gives a value of 7.08 (corresponding PCO2 : 110 mmHg). This technique causes hypercarbic cerebral vasodilation which induces luxuriant perfusion and makes the flow pressure dependent [22]; selfregulation is lost, and the risks of cerebral oedema or embolisation are increased. In the Trendelenburg position, intracranial pressure can increase dangerously when venous pressure is high and the arterial vessels are maximally vasodilated.
The alpha-stat technique, which is simpler in practice, is used routinely for ECC in moderate hypothermia (25-30°C), as neurological outcomes at 6 weeks and 2 months are significantly better in these conditions [23,26,31]. In addition to shifting the Hb dissociation curve to the right, the pH-stat strategy has the advantage of doubling the cerebral blood flow (CBF). As this improves the homogeneity of cooling and rewarming, the pH-stat technique is probably indicated during cooling and rewarming of patients brought to a low temperature (< 25°), as it promotes rapid and uniform brain thermal changes, especially during complete circulatory arrest [2,16]. In situations where selfregulation is impaired such as diabetes, hypertension and history of stroke, the pH-stat technique is probably preferable as it better maintains cerebral oxygenation [15]. Note that selfregulation is ineffective below 25°C anyway. For ECCs of less than 90 minutes and temperatures above 30°C, the two techniques do not differ significantly.
Proteins behave as weak acids at the usual pH of blood and are a major buffer system in the acid-base balance. In contrast to the bicarbonate system, they maintain their buffering capacity intact at low temperatures because the degree of dissociation of the imidazole group of histidine does not change in relation to water as a function of temperature. In hypothermia, the acidosis must therefore be corrected by the addition of protein, because the buffering capacity of bicarbonate becomes ineffective below 28°C [34].
| Hypothermic ECC |
|
Any decrease in temperature lowers metabolic demand by 7% per degree C. Main benefits of hypothermia (mild: 32-35°, moderate: 28-31°, deep: < 27°):
- Reduction of pump flow (1.8 L/min/m2 at 28°)
- Improved brain protection
- Improved myocardial protection in long or complex operations
Disadvantages of hypothermia :
- Increase in blood viscosity (compensated by haemodilution: Ht in % = T in °C)
- Increase in SAR
- Left shift of the Hb dissociation curve
- Coagulation disorders
- Need to warm the patient
Apart from circulatory arrest where it is crucial (T° 18-20°C), hypothermia is mainly beneficial to the brain and heart, but the latter is also well protected by warm ± continuous cardioplegia. With the exception of long, complex or circulatory arrest cases that clearly benefit from moderate or deep hypothermia, normothermic or mild hypothermic (T ≥ 34°) bypass surgery offers equivalent results and tends to be the norm for routine cases. Cerebral cooling of 2-4° already provides protection for the neurons. Normothermic cardioplegia improves the recovery of postoperative myocardial function and reduces the risk of arrhythmias.
It is essential to avoid cerebral hyperthermia during the warming up process. Warming should be slow and even:
- Heating rate: 1°C/5 min
- Maximum blood temperature in the arterial line: 37°.
- Max gradient between the arterial line and the oesophagus: 3°.
- Max gradient between blood T° and water T° (heat exchanger): < 10° when blood T° is < 30°, < 4° when blood T° is > 30°.
- Warming up to 36° - 36.5° (central T°)
Measurement of brain temperature: jugular venous blood (retrograde cannulation), tympanic probe, nasal probe against ethmoidal cells. Other sites may have a deviation of up to 5° from brain temperature.
The regulation of acid-base balance in hypothermia can be done in two ways, each with its own preferred indications:
- ∝ stat (constant CO content ): low risk cases, bypass surgery < 90 minutes, mild hypothermia
- pH-stat (constant pH): cooling and heating, high risk situations
altered cerebral self-regulation (hypertension, diabetes, history of stroke)
|
© CHASSOT PG, GRONCHI F, last update December 2019
References
- ADAMS DC, HEYER EJ, SIMON AE, et al. Incidence of atrial fibrillation after mild or moderate hypothermic cardiopulmonary bypass. Crit Care Med 2000; 28:309-11
- AOKI M, NORMURA F, STROMSKI ME, et al. Effects of pH on brain energetics after hypothermic circulatory arrest. Ann Thorac Surg 1993; 55:1093-1103
- AZMOON S, DEMAREST C, PUCILLO AL, et al. Neurologic and cardiac benefits of therapeutic hypothermia. Cardiol Rev 2011; 19:108-14
- BISSONNETTE B, HOLTBY HM, PUA DAJ, ET AL. Cerebral hyperthermia in children after cardiopulmonary bypass. Anesthesiology 2000; 93:611-8
- BORGER MA, RAO V. Temperature management during cardiopulmonary bypass: Effect of rewarming rate on cognitive dysfunction. Semin Cardiothorac Vasc Anesth 2002; 6:17-20
- COOK DJ. Con: Temperature regimens and neuroprotection during cardiopulmonary bypass: does rewarming rate matter? Anesth Analg 2009; 109:1733-7
- CROUGHWELL ND, NEWMAN NF, BLUMENTHAL JA, et al. Jugular bulb saturation and cognitive dysfunction after cardiopulmonary bypass. Ann Thorac Surg 1994; 58:1702-8
- ENGELMAN R, BAKER RA, LIKOSKY DS, et al. The Society of Thoracic Surgeons, the Society of Cardiovascilar Anesthesiologists, and the American Society of Extracorporeal Technology: clinical practice guidelines for cardiopulmonary bypass - Temperature management during cardioplmonary bypass. J Cardiothorac Vasc Anesth 2015; 29:1104-13
- FAN Y, ZHANG AM, XIAO YB, et al. Warm versus cold cardioplegia for heart surgery: A meta-analysis. Eur J Cardiothorac Surg 2010; 37:912-9
- GRIGORE AM, GROCOTT HP, MATHEW JP, et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg 2002; 94:4-10
- GRIGORE AM, MURRAY CF, RAMAKRISHNA H, DJAIANI G. A core review of temperature regimens and neuroprotection during cardiopulmonary bypass: does rewarming rate matter? Anesth Analg 2009; 109:1741-51
- GROCOTT HP, MACKENSEN B, GRIGORE AM, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke 2002; 33:537-41
- HESSEL EA. Cardiopulmonary bypass equipment. In: ESTEFANOUS FG, et al. Eds. Cardiac anesthesia. Principles and clinical practice, 2nd edition. Philadelphia, Lipincott Williams & Wilkins, 2001, 335-86
- HOGUE CW, PALIN CA, ARROWSMITH JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg 2006; 103:21-37
- HOOVER LR, DINAVAHI R, CHENG WP, et al. Jugular venous oxygenation during hypothermic cardiopulmonary bypass in patients at risk for abnormal cerebral selfregulation: influence ofa -stat versus pH-stat blood gas management. Anesth Analg 2009; 108:1389-93
- JONAS RA. Hypothermia, circulatory arrest, and the pediatric brain. J Cardiothorac Vasc Anesth 1996; 10:66-74
- JONES T, ROY RC. Should patients be normothermic in the immediate postoperative period? Ann Thorac Surg 1999; 68:1454-5
- JOSHI B, BRADY K, LEE J, et al. Impaired selfregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg 2010; 110:321-8
- KERN FH, UNGERLEIDER RM, REVES JG, et al. The effect of altering pump flow rate on cerebral blood flow and cerebral metabolism in neonates, infants and children. Ann Thorac Surg 1993; 58:1366-72
- MITCHENFELDER JD. The awake brain. In: Anesthesia and the brain. New York: Churchill-Livingstone, 1988, 7-8
- MITCHENFELDER JD, MILDE JH. The relationship among canine brain temperature, metabolism and function during hypothermia. Anesthesiology 1991; 75:130-6
- MURKIN JM, FARAR JK, TWEED WA. Cerebral selfregulation and flow/metabolism coupling during cardiopulmonary bypass: the influence of PaCO2 . Anesth Analg 1987; 66:825-32
- MURKIN JM, MARTZKE JS, BUCHAN AM, et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. II Neurologic and cognitive outcomes. J Cardiothorac Vasc Surg 1995; 110:349-55
- NUSSMEIER NA, CHENG W, MARINO M, et al. Temperature during cardiopulmonary bypass: The discrepancies between monitoring sites. Anesth Analg 2006; 103:1373-9
- PATEL PA, DESAI ND. Con: Cardiac surgery should be performed under warm conditions. J Cardiothorac Vasc Surg 2012; 26:949-51
- PATEL RL, TURTLE MR, CHAMBERS DJ, et al. Alpha-stat acid-base regulation during cardiopulmonary bypass improves neuropsychologic outcomes in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 1996; 111:1267-79
- REBEYKA I. Hypothermia. In: JONAS RA, ELLIOTT MJ. Cardiopulmonary bypass in neonates, infants and young children. Oxford: Butterworth, 1994, 54-66
- REES K, BERANEK-STANLEY M, BURKE M, et al. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Cochrane Database Syst Rev 2001; CD002138
- ROMAN PEF, GRIGORE AM. Pro: hypothermic cardiopulmonary bypass should be used routinely. J Cardiothorac Vasc Surg 2012; 26:945-8
- SANO T, DRUMMOND J, PATEL P, et al. A comparison of the cerebral protective effects of isoflurane and mild hypothermia in a model of incomplete forebrain ischemia in the rat. Anesthesiology 1992; 76:221-8
- SHANN KG, LIKOSKY DS, MURKIN JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg 2006; 132:283-90
- SVYATETS M, TOLANI K, ZHANG M, et al. Perioperative management of deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2010; 24:644-55
- SWAMINATHAN M, STAFFORD-SMITH M. Renal dysfunction after vascular surgery. Curr Opin Anaesthesiol 2003; 16:45-52
- TALLMAN RD. Acid-base regulation, alpha-stat, and the Emperor's new clothes. J Cardiothorac Vasc Anesth 1997; 11:282-8
- WATANABE T, HRITA H, KOBAYASHI M, et al. Brain tissue pH, oxygen tension and carbon dioxide tension in profoundly hypothermic cardiopulmonary bypass. J Thorac Cardiovasc Surg 1989; 97:396-401
- WOLBERG AS, MENG ZH, MONROE DM, et al. A systematic evaluation of temperature on coagulation enzyme activity and platelet function. J Trauma 2004; 56:1221-8
- YAU TM, CARSON S, WEISEL RD, et al. The effect of warm heart surgery on postoperative bleeding. J Thorac Cardiovasc Surg 1992; 103:1155-63