Air-blood contact with foreign surfaces (tubes, membranes, etc) requires deep anticoagulation to avoid the risk of circuit thrombosis (see Haematological aspects). Of the many anticoagulants in clinical use (Table 7.1 and Table 7.2), only unfractionated heparin (UFH) is routinely used in ECC. It is a part of it. For more details on coagulation and haemostasis in cardiac surgery, the reader may refer to Chapter 8 (Coagulation & Haemostasis).
Heparin
Unfractionated heparin (UFH) is a glycosaminoglycan (molecular weight 12,000-30,000) that binds to antithrombin III (AT III) and accelerates its ability to inhibit factors IIa (thrombin), IXa and Xa. Without heparin, thrombin and Xa are inhibited by AT III with a half-life of about 1 minute. In the presence of heparin, this reaction is extented 2,000 times. AT III also has indirect anti-inflammatory properties by acting on the production of prostaglandins. The high consumption of AT III during heparinisation may activate white blood cells and contribute to the post-ECC inflammatory cascade [30]. In high doses, heparin binds to and inhibits platelets [35].
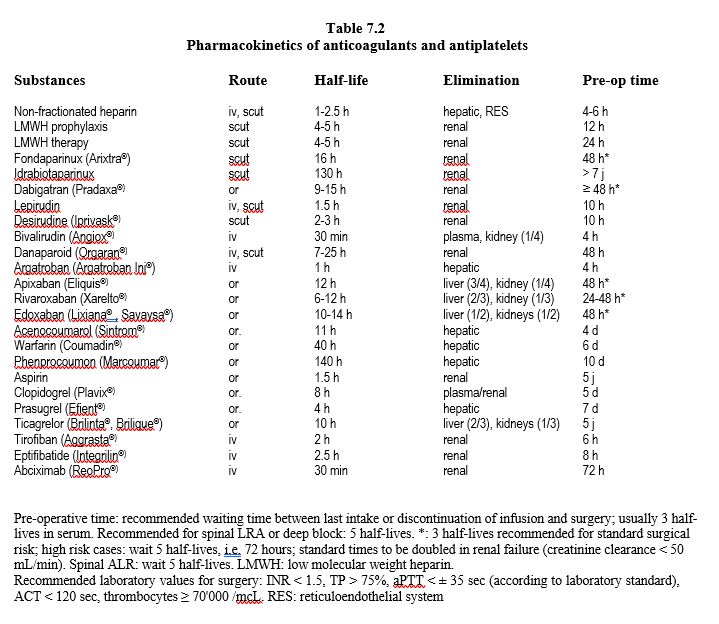
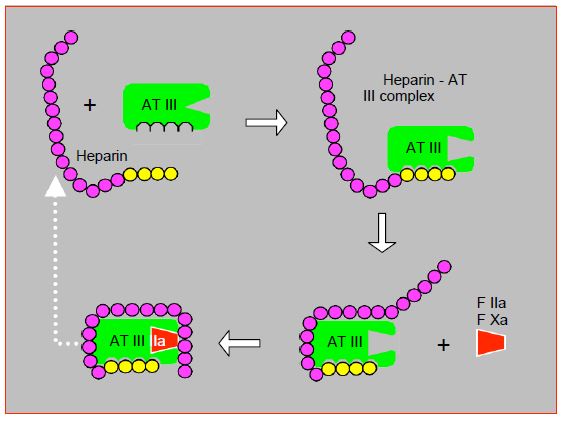
The action of heparin on AT III is twofold. Binding to the high-affinity fragment of heparin induces a change in the configuration of AT III which significantly increases its affinity for coagulation factors. The low affinity fragment brings the factor closer to the site of action of AT III. Once AT III has bound to the factor, a conformational change decreases the affinity for heparin, which is released and can bind to another AT III molecule (Figure 7.19A) [41].
These two different mechanisms explain the more or less selective actions of the different heparin derivatives. UFH has an anti-Xa:anti-IIa activity ratio of 1:1. Low molecular weight heparins (LMWH, MW 2'000-10'000) have an anti-Xa:anti-IIa ratio of 2-4:1 and the pentasaccharide fondaparinux selectively inhibits factor Xa. For AT III to deactivate thrombin, both mechanisms, conformational change and approximation, are equally important. For deactivation of Xa, on the other hand, approximation plays no role. LMWHs can, with their high affinity segment, induce the necessary conformational change, but their chain is too short to cope well with thrombin, which explains their weaker action against it. Fondaparinux matches the sequence of the high affinity segment which is the minimum chain length required to induce the conformational change, without having any bridging ability. Thus, unable to inhibit IIa, it selectively inhibits factor Xa [12].
UFH,LMWH and fondaparinux are poorly absorbed orally and must be administered parenterally. Intravenously, UFH has an immediate onset of action, whereas the derivatives have a 20-60 minute delay of action. The anticoagulant effect of UFH is highly variable due to strong protein and endothelium binding [24]. LMWH and fondaparinux are much less bound and show a more predictable action profile. Because of the inter-individual variation in its effect, UFH requires constant monitoring. Its anticoagulant activity correlates well with aPTT and ACT (activated clotting time). The shorter derivatives have no predictable effect on this parameter and are therefore more difficult to monitor, but monitoring is not necessary because their pharmacokinetic profile is more stable.
UFH is resorbed and metabolised by the endothelium and the reticuloendothelial system. The metabolites are inactive and are eliminated by the renal route. The biological half-life depends on the dose administered: 1 hour at 100 U/kg and 2.5 hours at 400 U/kg [7]. Hypothermia considerably slows down the clearance of heparin. However, a major limitation of UFH is its inability to inhibit fibrin-bound thrombin in a thrombus. Indeed, thrombin in microthrombi formed on the surfaces of the bypass graft is beyond the reach of heparin. Direct thrombin inhibitors (lepirudin, bivalirudin, argatroban) have been developed to better inhibit thrombin embedded in the thrombus, but their application in routine clinical practice is limited by their short half-life (30-60 minutes) and lack of antagonist. LMWH and fundaparinux, which also cannot be antagonised, do not have sufficient anti-IIa activity to be useful in ECC.
Heparin and ECC
UFH is essential for the smooth running of a bypass operation and is the basic anticoagulant. For this reason it is injected centrally after controlling the blood reflux, or administered directly into the RA by the surgeon; the injection is followed by a flush to ensure that the full dose is injected. The control ACT is measured 3-5 minutes later. The loading dose of heparin to achieve adequate anticoagulation for ECC (ACT ≥ 480 sec) is usually 300-400 IU/kg, but lower doses may sometimes be sufficient to achieve the desired ACT. An ACT > 480 sec is required with standard non-biocompatible circuits and use of cardiotomy suction. The desired circulating heparin concentration is 2-4 IU/mL. To determine the dose required in a given patient, it is sufficient to construct his dose-response curve obtained with 2 determinations of ACT: the baseline value and the value after mixing the blood in vitro with a known concentration of heparin (Figure 7.19B) [7]. The heparinemia test is preferred to the ACT test for regulating heparin administration [3].
Intravenously, UFH has an immediate effect. To compensate for the effect of haemodilution, 5,000-10,000 IU are added to the priming fluid of the bypass. These two doses are usually enough for 60-90 minutes of ECC. Subsequently, additional doses (50-100 IU/kg every 30-120 min) should be titrated according to the patient's individual response to heparin [35]. UFH is the most widely used thrombin inhibitor in ECC because of its ease of administration, reversibility by protamine, and the possibility of measuring its activity with coagulation tests.
Figure 7.19A: Mechanism of action of unfractionated heparin. After binding with its high affinity fragment for AT III (yellow fragment), heparin induces a conformational change at the AT III action site, which significantly increases its affinity for factors IIa (thrombin) and Xa. The low-affinity fragment of the heparin chain (purple) serves to bring factor IIa closer to the action site of AT III; this mechanism is not required for factor Xa binding. Once AT III has bound to factors IIa and Xa, a conformational change decreases the affinity for heparin, which is released and can bind to another AT III molecule (white dotted arrow).
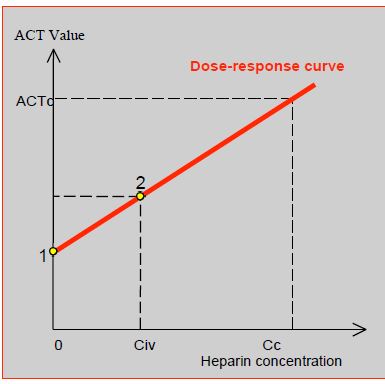
Figure 7.19B: Heparin dose response curve, constructed from a minimum of 2 points. 1: baseline ACT value before heparin injection. 2: ACT value after mixing with a given concentration of heparin (Civ: in vitro concentration). Extrapolation of the curve determines the amount of heparin (Cc: target concentration) that the patient needs to achieve an ACT of 480 seconds (ACTc: target ACT). The loading dose is calculated from the Cc with reference to the patient's estimated circulating volume [7].
High concentrations of heparin (≥ 300 IU/kg) induce the release of lipoprotein lipase and hepatic lipase, which hydrolyse plasma triglycerides into free fatty acids (FFA), the level of which rises by 15-20%. These free fatty acids bind to the plasma proteins and displace the substances bound to them, thus increasing the free concentration of these substances and thus their pharmacological activity. This process takes place within minutes [31].
Heparin activity is usually measured by ACT, the normal value of which is between 80 and 120 seconds. The minimum ACT necessary to avoid thrombotic or haemorrhagic problems during ECC is still debated, but a value of > 400 seconds is generally accepted as a reference for standard circuits (480-500 seconds), and a value of ≥ 300 seconds for assistants with pre-heparinised circuits [21,34]. If the ACT is < 400 seconds, ECC is not started without adding an additional dose of heparin (5,000 - 10,000 IU); a repeat test is performed after 3 minutes [13]. Two ACT tests are performed before starting ECC: before heparin injection (normal patient value), and 3-4 minutes after the heparin bolus [34].
Heparin resistance
Insufficient ACT prolongation after a standard dose of UFH (< 450 seconds for 500 U/kg) indicates patient resistance to heparin due to antithrombin deficiency (AT III activity < 60%). Several phenomena explain this insufficient response [7,8].
- Congenital AT III deficiency (incidence 1:3,000); AT III levels are reduced by 40-60%.
- Consumption of AT III by ongoing heparin therapy; the fall in antithrombin is 5-10% per day. Preoperative treatment with heparin for several days is the most frequent cause.
- Haemodilution; plasma AT levels may decrease by 30%.
- Decreased AT III production in liver failure, malnutrition or nephrotic syndrome.
- Increased AT III consumption in sepsis, DIVC, pulmonary embolism or mechanical circulatory support.
- Thrombocytosis; as heparin binds to platelets, an excess of platelets reduces the amount that can activate AT III.
Management is based on three elements.
- Increase in heparin doses. However, there is a ceiling effect: anticoagulation no longer deepens when the heparin level is > 4 U/mL [22].
- Antithrombin concentrate, in purified or recombinant human form. The recommended dose is 500-1,000 IU for an adult [42], which is relatively modest, as up to 45 U/kg is required to maintain normal ATIII levels [5]. The cost of this treatment is CHF 1,200-2,500, but it is much more effective than FFP.
- Fresh thawed plasma. FFP contains approximately 1 U of AT III per mL. Two bags of FFP are rarely enough to compensate for AT III deficiency [5]. The administration of enough AT III to normalise AT III (1-2 L) induces the danger of hypervolaemia. On the other hand, FFP carries all the infectious and allergic risks associated with blood element transfusions.
The most logical approach is to measure ATIII activity (target ≥ 80%) and only give antithrombin concentrate when the level is low; if it is normal, an increase in heparin dosage is usually enough [7].
Alternatives to heparin
In patients with heparin-induced thrombocytopenia (HIT) it is still possible to perform ECC with other anticoagulants if it is not possible to postpone the procedure until the anti-heparin-PF4 antibodies are negative (see Chapter 8 HIT). These substances are direct thrombin blockers, which inhibit both circulating and fibrin-bound thrombin, whereas heparin has no impact on the latter. However, they have no antagonist, and are not reversed by protamine. Their elimination depends on their serum half-life (Tables 7.1 and 7.2) and the use of a haemofiltration circuit [1,4,8,15,18,35].
- Bivalirudin (Angiox® , Angiomax® ): reversible direct thrombin inhibitor. Onset of activity in 2-10 minutes; half-life: 25 min (doubled in renal failure); renal elimination. Easiest to handle for ECC, but with risk of thrombosis in the reservoir, oxygenator or coronary bypass if machine flow is interrupted, as elimination of bivalirudin by plasma proteolysis continues in still blood. Biocompatible circuits, arteriovenous shunts, a CellSaver™ on blood recovery, intermittent flushing of the venous reservoir and storage of blood in citrated bags are therefore required. Bivalirudin linearly increases ACT, TP, PTT and TT; the most reliable test is the ecarin clotting time (ECT), but it is not feasible in all laboratories. Dosage: bolus 1 mg/kg + 50 mg in the ECT priming fluid + infusion 2.5 mg/kg/hr. ACT ≥ 450 sec is targeted with additional boluses of 0.1-0.5 mg/kg [17]. The desired ECT is 400-500 sec, corresponding to a serum level of 10-15 mcg/mL [18,33]. The infusion is stopped 10-15 minutes before the end of the ECT. Ultrafiltration is only used after the bypass to accelerate the elimination of bivalirudin. A return to the pump is impossible after weaning because of the risk of circuit thrombosis.
- Argatroban (Argatroban Injection® ): synthetic molecule that selectively and reversibly binds to thrombin. Half-life: 40-50 minutes; hepatic elimination. Dosage: bolus 0.1-0.2 mg/kg iv + 0.05 mg/kg in ECC priming fluid + infusion 5-10 mcg/kg/min (immediate and continuous) for ACT > 400 sec and aPTT 3 times baseline; additional boluses if necessary: 2 mg. However, correlation between serum levels and ACT is inconsistent. Return to normal coagulation 2-4 hours after stopping the infusion. Argatroban is the drug of choice in cases of renal dysfunction.
- Danaparoid sodium (Orgaran® ): Predominantly inhibits factor Xa. Half-life of 7 hours for anti-IIa activity and 25 hours for anti-Xa activity; renal elimination. Dosage: bolus iv 1,500-2,000 U + 5,000-10,000 U in the ECC priming fluid; add 1,500 U after 2 hours. Very difficult to manage on bypass and very haemorrhagic postoperatively; virtually abandoned.
- Lepirudin (Refludan® ): recombinant form of hirudin, irreversible thrombin inhibitor. Serum half-life 10 min, elimination half-life 1-1.5 hours, but irreversible thrombin inhibition; renal elimination. Best monitored by ECT (ecarin clotting time of citrated whole blood). Dosage: bolus 0.25 mg/kg + 0.2 mg/kg in ECT priming fluid + bolus 5 mg for ACT > 350 sec (imprecise); infusion 0.15 mg/kg/h. Lepirudin production ceased in 2012 for commercial reasons.
- Desirudin (Iprivask® ): a molecule very similar to lepirudin used for percutaneous coronary interventions. No experience with ECC.
An alternative approach is to anticoagulate patients with heparin, and at the same time block platelet aggregation induced by the action of preformed IgG antibodies on PF4-heparin complexes with a short-acting antiplatelet agent. Although still responsive to heparin, patients are no longer at excessive risk of thromboembolism and thrombocytopenia [27].
- Heparin (usual dose) + prostacyclin (Iloprost® ): increases intraplatelet cAMP and inhibits activation. Half-life 15-30 min. Infusion of 3-12 ng/kg/min (avg. 7.6 ng/kg/min), set according to the HIPA (Heparin-induced platelet aggregation) test for activation < 5% [2]. The infusion is halved after protamine and reduced to one quarter on arrival in the ICU and stopped 1 hour later [27]. Disadvantages: iloprost causes hypotension (regulated by norepinephrine), and intraoperative HIPA tests are time consuming (30-40 minutes).
- Heparin (bolus 5,000 IU and infusion 1,000 IU/h) + tirofiban (Aggrastat® ): blocker of the GP IIb/IIIa receptor of platelets, responsible for the binding of platelets with fibrinogen (see Figures 8.12 and 8.13). Half-life: 2 hours. Bolus 0.4 mcg/kg, then infuse 0.15 mcg/kg/hour. Stop 1 hour before the end of the bypass [19].
Protamine
Protamine, originally extracted from salmon sperm, is now produced by recombinant technology. It is a positively charged polycationic molecule that forms stable complexes with heparin, which is negatively charged. The plasma half-life of protamine is 7 minutes, while that of heparin is 60-120 minutes [8]. One mg of protamine (100 IU) neutralises 1 mg of heparin (100 IU). Usually a protamine dose of 80% of the heparin dose is given, but dosing based on the patient's actual heparin level or dose-response curve (Hepcon™ or HMS™ systems) leads to lower amounts being given and thus reduces platelet dysfunction, bleeding and the risk of transfusions after CEC [34,39]. An ACT done 5 minutes after the end of protamine administration should be within ±10% of the pre-ECC baseline value.
Protamine has several immediate side effects, the incidence of which varies from 1 to 13% of cases (mean 2.6%) [16,23].
- Histamine release, which is characterised by significant vasodilatation with a decrease in preload and afterload; it is directly proportional to the rate of administration (type I reaction) and decreases in intensity if the protamine is diluted in 100 mL 0.9% NaCl for each half dose. It is very often necessary to accelerate the infusions and to give a vasoconstrictor (neosynephrine or noradrenaline) to counteract this effect.
- Pulmonary hypertension; heparin-protamine complex triggers the release of thromboxane A2, which is a pulmonary vasoconstrictor; PAP rises by 25-50%.
- Antigen-antibody reaction (IgG and IgE) (type II reaction); rarer, this occurs in re-operated patients who have previously received protamine and in diabetics treated with insulin stabilised with protamine (protamine-Zn); the phenomenon is possible in patients allergic to fish, and in vasectomized men.
- Anaphylactoid reaction triggered by the heparin-protamine complex by directly activating the classical complement pathway (C4a) without the intermediary of an antigen-antibody reaction (type III reaction, which occurs in 1.5% of cases). The clinical picture is that of a catastrophic anaphylactic reaction; the massive release of thomboxane triggers pulmonary hypertension and bronchoconstriction which can be devastating and force a return to ECC. As this reaction is related to NO, methylene blue may be effective but it may worsen the pulmonary hypertensive crisis [38].
- In excess of heparin (> 1.5:1 mg), protamine has an anticoagulant effect: it inhibits platelet function, prolongs TCA and increases bleeding [21,34,39]. Blood loss increases by 26% when the heparin:protamine ratio is 1.3 instead of 0.8 [25].
Allergic patients can be operated with heparinised circuits and reduced systemic heparinisation (100 IU/kg), thus avoiding the administration of protamine [26]. A steroid dose during ECT (500 mg methylprednisolone) and a very slow injection (infusion over 20 minutes) via a peripheral route may decrease the intensity of the reaction in an allergic patient. It is recommended to wait for the effect of protamine and to perform a test (thromboelastogram, ROTEM™, HMS™) before administering other agents intended to improve blood coagulation such as hemoconcentrated autologous blood, isolated coagulation factors, PFC or platelets. The volume that the perfusionist retrieves from the circuits after the ECC is a perfusate containing heparin; its haematocrit is the same as that of the ECC, therefore less than 30%.
The administration of protamine is started after venous decanulation of the RA. Usually the first half of the dose is injected slowly (maximum 50 mg/min) peripheral intravenously , then the aortic cannula is removed and the second half of the protamine is given. This reduces bleeding during arterial decanulation and avoids thrombus formation in the aorta, and allows a quicker return to ECC in case of major problems. If the arterial cannula is in the femoral or subclavian position, protamine is not started until circulation in the artery is fully restored. As soon as protamine administration has started, the aspirations on the ECC machine are stopped and the lost aspirations and CellSaver™ are switched; this is because clots can form in the reservoir and oxygenator, requiring the circuit to be changed in case of returning on pump for an acute problem.
| Anticoagulation in ECC |
|
Contact of the blood with foreign surfaces and air requires complete anticoagulation with unfractionated heparin (UFH). Heparin is administered centrally prior to ECC at 300-400 U/kg to achieve an ACT > 400 seconds. If the ACT is < 400 seconds, an additional dose of 10,000 U is added and rechecked. If the ACT cannot be prolonged despite an adequate dose of UFH, antithrombin III deficiency should be suspected and replaced with concentrate (800 - 1000 U/kg) or fresh frozen plasma. The desired ACT during ECC is ≥ 480 seconds (standard circuits, cardiotomy aspiration).
Patients with heparin-induced thombocytopenia may be anticoagulated with other substances, unfortunately without antagonists and not reversible with protamine:
- Lepirudin (Refludan® )
- Danaparoid sodium (Orgaran® )
- Argatroban (Argatroban Injection® )
- Bivalirudin (Angiox® )
- Tirofiban or prostacyclin + heparin
Protamine antagonises unfractionated heparin at a ratio of 1 mg (100 IU) to 1 mg of heparin (100 IU). The administration of protamine is started after decanulation of the RA; half of the intended dose is given before decanulation of the aorta and the second half after decanulation of the aorta. As soon as the administration of protamine has begun, the aspirations of the ECC machine are interupted.
Protamine has several side effects:
- Systemic vasodilation and arterial hypotension
- Pulmonary vasoconstriction
- Antigen-antibody reaction
- Sudden anaphylactoid reaction (anaphylactic shock)
|
Intraoperative coagulation tests
The development of point-of-care tests (POC tests) that can be performed at the patient's bedside within 10 minutes provides a more accurate picture of the coagulation status and the degree of anticoagulation (see Chapter 8, Intraoperative coagulation tests). This allows for the administration of different haemostatic factors to be prioritised according to the specific deficits detected, rather than infusing various coagulation agents indiscriminately. Three types of tests are used during ECC procedure.
- Activated clotting time (ACT);
- Clot viscoelastic strength tests (TEG™, ROTEM™, Sonoclot™);
- Platelet aggregability tests (Multiplate™, VerifyNow™, PFA-100™, etc).
The most widely used is the ACT (Activated clotting time) because it quantifies the degree of heparinisation of the patient: blood is placed in a container that contains an activator of the intrinsic pathway (celite, kaolin, glass or a combination of these), and the tube is placed in a machine that heats the blood to 37°C and measures the clotting time. Although it has a near linear relationship with serum heparin levels, ACT is not specific for heparin, and may show some variability (± 10% depending on the technique) [32]. Several factors prolong ACT independently of heparin: coagulation factor, fibrinogen or platelet deficiency, haemodilution, hypothermia, excess protamine, anti-phospholipid antibodies, lupus [35].
In addition to ACT, heparin can be monitored by measuring its circulating concentration in two different ways [21].
- Titration with a known dose of protamine (1 IU of protamine neutralises 1 IU of heparin) (Hepcon™ system); independent of haemodilution.
- Fluorometric titration of anti-Xa/IIa activity; independent of the amount of antithrombin. This test is the gold standard, but it is neither rapid nor feasible at the patient's bed.
The measurement of heparin concentration is more reliable than ACT, as it correlates better with anti-Xa activity than the latter; ACT tends to overestimate the actual activity of heparin, thus under-anticoagulating the patient [34].
The thromboelastogram (TEG™, ROTEM™) measures a series of parameters corresponding to the viscoelastic properties of the thrombus under a shear force corresponding to that in the venous circulation [36].
- Clotting time;
- Clot formation time;
- Speed of fibrin formation;
- Maximum clot firmness;
- Maximum lysis of the clot.
The addition of different reagents allows differentiation between the need for protamine, antifibrinolytic, fibrinogen, coagulation factors or thrombocytes (see Figure 8.18). The thromboelastogram is not yet validated or standardised as a measure of heparin, but it is useful for individualised assessment of the most appropriate heparin/protamine ratio [6,34].
The thromboelastogram is not designed to assess platelet function. Several tests are available to assess the latter, but they assess different mechanisms of thrombocyte function and their degree of consistency is modest (Multiplate™, VerifyNow™, PFA-100™, etc) [28]. However, they are useful in patients on antiplatelet therapy, in whom they have a better predictive value than the time between surgery and the last clopidogrel dose [20,29]. They allow better management of fresh platelet transfusion or desmopressin administration in case of refractory bleeding [37].
The use of these tests in algorithms (see Figure 8.19) based on targeted administration of procoagulants saves blood transfusions (-10% to -62%), platelet transfusions (-25%) and especially fresh thawed plasma (5-10 times less); it halves the incidence of massive transfusions and the rate of surgical re-exploration for haemostasis; and it lowers morbidity (renal failure, sepsis, thrombosis) and the incidence of thrombo-embolic events. However, it doubles the use of fibrinogen and prothrombin complex concentrate and tends to increase platelet transfusions [9,10,14,40]. The thromboelastogram is performed at aortic declamping, prior to weaning, so that the specific products are available as soon as the protamine has been injected; it is then repeated checking treatment efficiency or to correct it as necessary [11]. However, the correlation between test results and bleeding risk is not linear; normal values do not preclude the need for transfusion or procoagulation [6]. This is because the endothelial component of coagulation is not fully accounted for in these tests and the effect of antiplatelet drugs is not detected.
| Coagulation tests in ECC |
|
ACT is the most commonly used measure to assess the degree of heparinisation (target value: ≥ 480 sec). It is performed every 20-30 minutes during the course of the bypass procedure.
The thromboelastogram can differentiate between the need for protamine, antifibrinolytics, fibrinogen, clotting factors or thrombocytes and improves the management of bleeding if used as part of a decision algorithm.
A platelet function test is useful in patients on anti-platelet therapy or in cases of refractory bleeding.
|
© CHASSOT PG, GRONCHI F, April 2008, last update December 2019
References
- ADAM RLC, BIRD RJ. Review article: Coagulation cascade and therapeutic update: Relevance to nephrology. Part I: Overview of coagulation, thrombophilia and history of anticoagulants. Nephrol 2009; 14:462-70
- ANTONIOU T, KAPETANAKIS EI, THEODORAKI K, et al. Cardiac surgery in patients with heparin-induced thrombocytopenia using preoperatively determined dosages of Iloprost. Heart Surg Forum 2002; 5:354-7
- BOER C, MEESTERS MI, MILOJEVIC M, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018; 32:88-120
- COPPENS M, EIKELBOOM JW, GUSTAFSSON D, WEITZ JI. Translational success stories. Development of direct thrombin inhibitors. Circ Res 2012; 111:920-9
- DIETRICH W, BUSLEY R, SPANNAGL M, et al. The influence of antithrombin substitution on heparin sensitivity and activation of hemostasis during coronary artery bypass graft surgery: a dose-finding study. Anesth Analg 2013; 116 :1223-30
- FABBRO M, WINKLER AM, LEVY JH. Technology: is there sufficient evidence to change practice in point-of-care management of coagulopathy? J Cardiothorac Vasc Anesth 2017; 31:1849-56
- FINLEY A, GREENBERG C. Heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg 2013; 116:1210-22
- GARCIA DA, BAGLIN TP, WEITZ JI, et al. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e24S-e43S
- GÖRLINGER K, DIRKMANN D, HANKE AA; et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decrease allogeneic blood transfusion in cardiovascular surgery. Anesthesiology 2011; 115: 1179-91
- GÖRLINGER K, FRIES D, DIRKMANN D, et al. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother 2012; 39:104-13
- GRONCHI F, RANUCCI M. Perioperative coagulation in cardiovascular surgery. In: MARCUCCI C, SCHOETTKER P, editors. Perioperative hemostasis. Coagulation for anesthesiologists. Heidelberg: Springer Verlag, 2014, 243-66
- HERBERT JM, HERAULT JP, BERNAT A, et al. Biochemical and pharmacological properties of SANORG 340006, a potent and long acting synthetic pentasaccharide. Blood 1998; 91:4197-205
- JOBES DR. Safety issues in heparin and protamin administration for extracorporeal circulation. J Cardiothorac Vasc Anesth 1998; 12:17-20
- KARKOUTI K, CALLUM J, WIJEYSUNDERA D, et al. Point-of-care hemostatic testing in cardiac surgery. A stepped-wedged clustered randomized controlled trial. Circulation 2016; 134:1152-62
- KELTON JG, ARNOLD DM, BATES SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N Engl J Med 2013; 368:737-44
- KIMMEL SE, SEKERES M, BERLIN JA, et al. Mortality and adverse events after protamine administration in patients undergoing cardiopulmonary bypass. Anesth Analg 2002; 94:1402-8
- KOSTER A, DYKE CM, ALDEA G, et al. Bivalirudin during cardiopulmonary bypass in patients with previous or acute heparin-induced thrombocytopenia and heparin antibodies: Results of the CHOOSE-ON trial. Ann Thorac Surg 2007; 83:572-7
- KOSTER A, FARAONI D, LEVY JH. Argatroban and bivalirudin for perioperative anticoagulation in cardiac surgery. Anesthesiology 2018; 128:390-400
- KOSTER A, KUKUCKA M, BACH F, et al. Anticoagulation during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II and renal impairment using heparin and the platelet glycoprotein Iib/IIIa antagonist tirofiban. Anesthesiology 2001; 94:245-51
- KWAK YL. KIM JC, CHOI YS, et al. Clopidogrel responsiveness regardless of the discontinuation date predicts increased blood loss and transfusion requirement after off-pump coronary artery bypass graft surgery. J Am Coll Cardiol 2010; 56:1994-2002
- LANDER H, ZAMMERT M, FITZGERALD D. Anticoagulation management during cross-clamping and bypass. Best Pract Res Clin Anaedsthesiol 2016; 30:359-70
- LEVY JH, MONTES F, SZLAM F, et al. The in vitro effect of antithrombin III on the activated coagulation time in patients on heparin therapy. Anesth Analg 2000; 90: 1076-9
- LOWENSTEIN E, ZAPOL WM. Protamine reactions, explosive mediator release and pulmonary vasoconstriction. Anesthesiology 1990; 73:373-8
- MANSON L, WEITZ JI, PODOR TJ, et al. The variable anticoagulant response to unfractionated heparin in vivo reflects binding to plasma proteins rather than clearance. J Lab Clin Med 1997; 130:649-655
- MEESTERS MI, VEERHOEK D, DE LANGE F, et al. Effect of high or low protamine dosing on postoperative bleeding following heparin anticoagulation in cardiac surgery. Thromb Haemost 2016; 116:251-61
- MUKADAM ME, PRITCHARD D, RIDDINGTON D, et al. Case 7-2001. Management during cardiopulmonary bypass of patients with presumed fish allergy. J Cardiothorac Vasc Anesth 2001; 15:512-9
- PALATIANOS G, MICHALIS A, ALIVIZATOS P, et al. Perioperative use of iloprost in cardiac surgery patients diagnosed with heparin-induced thrombocytopenia-reactive antibodies or with true HIT (HIT-reactive antibodies plus thrombocytopenia): an 11-year experience. Am J Hematol 2015; 90:608-17
- PANICCIA R, PRIORA R, LIOTTA A, et al. Platelet function tests: a comparative review. Vasc Health Risk Manag 2015; 11:133-48
- RANUCCI M, BARYSHNIKOVA E, SORO G, et al. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann Thorac Surg 2011; 91:123-30
- RANUCCI M, CAZZANIGA A, SORO G, et al. The anti-thrombin III saving effect of reduced systemic heparinization and heparin-coated circuits. J Cardiothorac Vasc Anesth 2002; 16:316-20
- ROSEN D, ROSEN K. Elimination of drugs and toxins during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997; 11:337-40
- ROZENTAL T, SHORE-LESSERSON L. Pharmacologic management of coagulopathy in cardiac surgery: An update. J Cardiothorac Vasc Anesth 2012; 26:660-79
- SALTER BS, WEINER MM, TRINH MA, et al. Heparin-induced thrombocytopenia. A comprehensive clinical review. J Am Coll Cardiol 2016; 67:2519-32
- SHORE-LESSERSON L, BAKER RA, FERRARIS VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines - Anticoagulation during cardiopulmonary bypass. Anesth Analg 2018; 126:413-24
- SNIECINSKI RM, LEVY JH. Anticoagulation management associated with extracorporeal circulation. Best Prac Res Clin Anaesthesiol 2015; 29:189-202
- THAKUR M, AHMED A. A review of thromboelastography. Int J Periop Ultrasound Appl Technol 2012; 1: 25-9
- VALGIMIGLI M, BUENO H, BYRNE RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J 2018; 39:213-54
- VIARO F, DALIO MB, EVORA PR. Catastrophic cardiovascular adverse reactions to protamine are nitric oxide/cyclic guanosine monophosphate dependent and endothelium mediated: should methylene blue be the treatment of choice? Chest 2002; 122:1061-6
- WANG J, MA HP, ZHENG H. Blood loss after cardiopulmonary bypass, standard vs titrated protamine: a meta-analysis. Neth J Med 2013; 71:123-7
- WEBER CF, GÖRLINGER K, MEININGER D. Point-of-care testing: a prospective randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012; 117: 531-47
- WEITZ JI, HIRSH J. New anticoagulant drugs. Chest 2001; 119:95s-107s
- WILLIAMS MR, D'AMBRA AB, BECK JR, et al. A randomized trial of antithrombin concentrate for treatment of heparin resistance. Ann Thorac Surg 2000; 70:873-7