Weaning off ECC is a critical moment that must be carried out in synergy with surgeon, perfusionist and anaesthetist. The therapeutic plan is discussed and established in advance, so as to avoid contradictory discussions and loss of time precisely when rapid decisions are needed. Communication is of utmost importance. All manoeuvres are coordinated and each person announces aloud the manoeuvres they are undertaking (see Chapter 4, Weaning off ECC).
The surgical field and the TEE display provide direct knowledge of heart's function chambers and filling.
- Through the sternotomy, the RA and the RV are observed; this allows the heart rhythm (synchronous rhythm, AF, complete AV block), the volume and degree of distension of the right chambers, and the contractility of the free wall of the RV to be assessed. The RV is dilated if it extends above the pericardial incision line at the level of the diaphragm.
- On TEE, global and segmental LV function, chamber volume, degree of hypo- or hypervolaemia, possible ventricular dilation and the presence of MI or TI are assessed. Functional indices of the LV and the RV are measured; valve function is monitored (prostheses, residual leak, paravalvular leak). Mitral flow provides information on rhythm (E and A flow or single flow) and on the efficiency of atrial contraction (importance of the A component).
The more impaired the myocardial function, the slower the weaning and the more gradual the weaning should be, assisting the heart at each 0.5-1 L/min increment for 5-10 minutes; the duration of this assistance is usually 10% of the aortic clamp time. Any failure should cause the pace to be slowed and inotropic support adjusted. Any right or left ventricular dilation is a sign of excessive overload. The onset or worsening of MI or TI is an excellent indicator of impending failure [2,3]. There are four stages to the actual weaning process.
- The first step is to lower the venous return to the bypass reservoir so as to permit some filling of the RA. This volume will be ejected by the RV and used as preload to the LV. Cardiac output increases as its preload increases (progressive increase in CVP and PCWP). TEE is a better guide to left filling than PCWP because the ventricle suffers from some diastolic dysfunction after ECC (myocardial oedema, cardioplegia, mechanical manipulation); under these conditions, the same filling volume corresponds to a higher pressure. TEE also provides information on ventricular size, function and valve performance.
- The second step is to progressively decrease the flow through the aortic cannula from 0.5 to 1.0 L/min. CVP and PCWP should remain at normal values; their elevation indicates a mismatch between filling and ventricular function.
- When hemodynamics are stable (BPsyst 80-100 mmHg, normal PAP) and the pump has been running at 1.0 L/min for at least 1 minute, the perfusionist interrupts the pump and clamps the venous and arterial cannulas.
- If the situation remains stable (APsyst > 90 mmHg, CI > 2.0 L/min/m2 , satisfactory TEE image), the venous cannula(s) are removed from the RA. Arterial decanulation takes place after the first half of protamine is injected (see Administration of protamine).
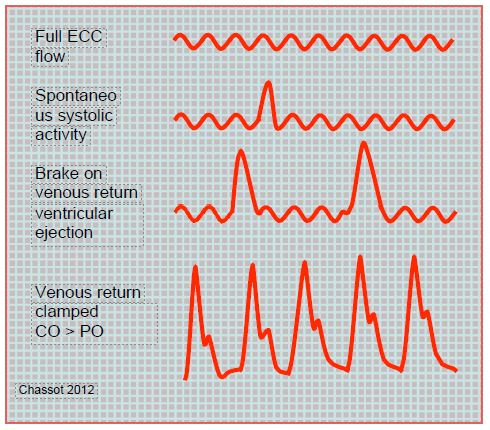
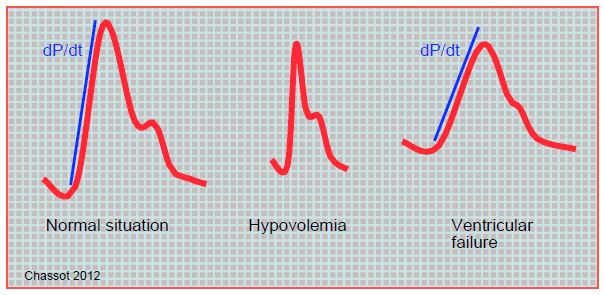
The shape of the arterial curve provides information on the gradual increase in cardiac output and also provides information on inotropic function (dP/dt) and blood volume ejection (area under the curve) (Figure 7.46 and Figure 7.47).
Figure 7.46: Schematic illustration of the arterial curve during weaning. Initially, flow is provided by the bypass machine, the heart shows only a few systolic increases. As the venous return to the machine is slowed down, the ventricle preload increases, allowing it to start ejecting. As the pump output decreases, the heart provides an increasing share of the cardiac output until the bypass pump is completely stopped. CO: cardiac output, PO:pump output
Figure 7.47: Schematic illustrations of the blood pressure curve in different circumstances. The blue line is the upward slope of the pressure curve, which is proportional to ventricular dP/dt. In hypovolaemia, the curve is narrow and sharp; the area under the curve, which is proportional to the stroke volume, is very small. In ventricular dysfunction, the upward slope is small, the curve is rounded and flattened. These analogue data are independent of the measured pressure value. They are more apparent on a femoral artery curve than on a radial artery curve. It goes without saying that these morphologies are only visible on the scope if the amplification is correct.
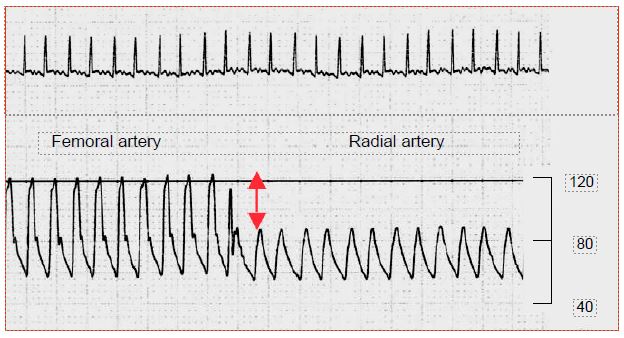
The pressure measured in the radial artery catheter may be much lower than the aortic artery pressure. The pulsatility of the curve is frequently lower. The displayed value of the radial, which is a muscular peripheral artery, may be 25-50% lower than that of the femoral artery, which is an elastic central artery. This occurs in 33-45% of cases, mainly in small patients, after hypothermic (< 32°C) bypass surgery or long aortic clamping, and when arterial vasoconstrictors are used [1,4]. The femoral catheter gives much more reliable information in these particular haemodynamic conditions (Figure 7.48).
Figure 7.48: Switching from a femoral to a radial artery artery recording. A drop in value of > 40% is explained by the intense peripheral arterial vasoconstriction after deep hypothermia. The femoral artery, which is an elastic artery like the aorta, is not influenced by this phenomenon specific to muscular arteries. Scale in mmHg.
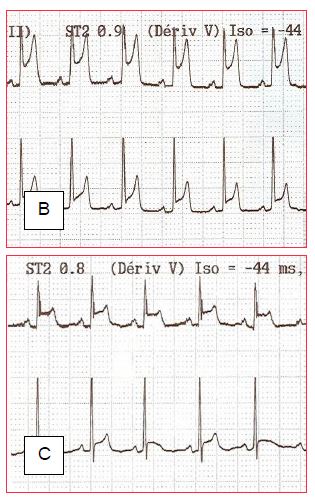
Sudden ST segment elevations are not uncommon. They have several causes and involve different treatments (Figure 7.49):
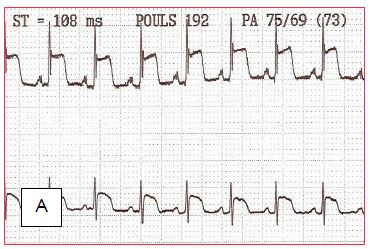
Figure 7.49: ST segment changes on discharge from bypass surgery (leads DII and V5). A: ST segment elevation secondary to coronary gas embolism. B and C: Progressive normalisation of the ST segment as the bubbles are fragmented and removed.
- Emboli: air bubbles (transient changes), atheromatous fragments (especially in thrombendarterectomy). Increase coronary perfusion pressure and inotropic effect to break up and remove air; atheromatous emboli are likely to be permanent.
- Technical problem with a graft, incomplete revascularisation; need to return to ECC and redo or complete bypasses; TEE is essential to objectify alterations in segmental kinetics (ACS Video).
- Infarction, most often secondary to thrombendarectomy.
- Arterial spasm (mammary artery on the IVA); treatment is diltiazem infusion.
Video : antero septo apica lakinesia mid oesophagal view 4 chambers (septum, antero apical area, lateral wall)
Video : antero septo apica lakinesia mid oesophagal view 2 chambers 90° (inferior wall, apex, anterior wall)
Video : antero septo apica lakinesia mid oesophagal view long axis 120° (posterior wall, apex, antero septal wall)
Video : Mid ventricular and apical akinesia of the LV (4 chambers 0° view) leading to cardiogenic schock and VAD; dilated LV poor function only basal part is contractile
Video : Anterior wall akinesia mid oesophagus view 90°
A sub-ST deviation is more indicative of subendocardial distress; it triggers an increase in perfusion pressure (noradrenaline) and the administration of nitrates.
After weaning but before decanulation, a 15-20 minute period of haemofiltration is commonly performed, which involves interposing a device consisting of semi-permeable microporous fibres contained in a cylinder into the ECC circuit. This system filters water, electrolytes and smaller proteins (see Haemofiltration and Figure 7.14). It can remove up to 180 mL per minute (4-5 L H2 O per hour) of water volume at a flow rate of 500 mL/min. Platelets, albumin and coagulation factors are retained, while water-soluble inflammatory mediators (TNF, IL-6, IL-8, IL-10) and complement (C3a, C5a) are removed with the discarded haemofiltrate. Heparin is partially filtered out [5].
Judicious pre-emptive use of catecholamines, which are started early enough (but not before aortic declamping), allows their therapeutic level to be reached at the time of weaning. It is possible to come off pump without amines if ventricular function is good (TEE check), BP normal and rhythm regular, but the situation is very progressive (progressive decrease of function until 6th hours post-ECC) and the inotropic need must be constantly reassessed. Under no circumstances should ventricular dysfunction and low cardiac output be allowed to develop. However, inotropic agents should be used sparingly, at the lowest rates and for the shortest duration necessary to maintain normal output. In the event of refractory hypotension (BPsyst < 80 mmHg), low output (CI < 1.5 L/min/m2 ), ventricular dilatation (TEE), new major MI, SvO2 < 55% despite maximum therapy, return to ECC for circulatory support, possible surgical correction, and placement of intra-aortic counterpulsation (IABP), ECMO, or ventricular support.
| Weaning off |
| Progressive clamping of venous return to the bypass reservoir and simultaneous reduction in pump flow
The poorer the ventricular function, the slower and more gradual the weaning should be. Assist support as needed
Special monitoring: MAP, VS (Swan-Ganz, PiCCO), PAP, SpO2 , SvO2 ,TEE
Assessment of ventricular function and volume in the surgical field (RV), on TEE (LV) and by arterial curve (radial artery can be buffered by vasoconstriction, femoral artery is more reliable)
Continuous adjustment of inotropic support to functional needs.
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- BOUCHARD-DECHÊNE V, COUTURE P, SU A, et al. Risk factors for radial-to-femoral artery pressure gradient in patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2018; 32:692-8
- DENAULT AY, BUSSIERES J, CARRIER M, et al. The importance of difficult separation from cardiopulmonary bypass: the Montreal and Quebec Heart Institute experience. Exp Clin Cardiol 2006; 11:37
- DENAULT AY, DESCHAMPS A, COUTURE P. Intraoperative hemodynamic instability during and after separation from cardiopulmonary bypass. Semin Cardiothorac Vasc Anesth 2010; 14:165-82
- FUDA G, DENAULT A, DESCHAMPS A, et al. Risks factors involved in central-to-radial arterial pressure gradient during cardiac surgery. Anesth Analg 2016; 122: 624-32
- SEARLES B, DARLING E. Ultrafiltration in cardiac surhery. In: MONGERO LB, BECK JR (eds). On bypass. Advanced perfusion techniques. Totowa (NJ, USA): Humana Press 2010, 193-210