Post-ECC neurological sequelae
Unfortunately, cardiac surgery carries a major neurological risk, which has long been attributed to ECC alone. Postoperative neurological disorders are usually classified into two categories.
- Type I includes focal lesions (stroke, TIA) and anoxic encephalopathy (coma);
- Type II consists of diffuse neuropsychological sequelae (deterioration of cognitive functions, memory and attention disorders, delays in psychomotor activity, delirium, convulsions) without signs of focalization.
Their prevalence varies greatly depending on how they are identified: clinical status, neuro-psychological tests, functional MRI, presence or absence of control groups in incidence studies (see Chapter 23 Neurological Complications and Risk Factors).
The incidence of type I neurological lesions is 1-5% (mean 2%), ranging from 1.6% in simple coronary artery bypass grafting, to 3-6% in aortic valve replacement and up to 17% in combined coronary and carotid surgery [63,72]. Their mortality is 21%. Neuropsychological dysfunction is much more common (1-34%), but is reversible in 80% of cases [9,53]. The great variability in the incidence of type II sequelae is due to the nature and disparity of the tests used and their highly variable sensitivity.
The major risk factors for type I neurological complications are patient-associated factors: ascending aortic atheromatosis, history of stroke, carotid stenosis, peripheral vasculopathy, diabetes, hypertension and advanced age (>75 years) [52,54]. The incidence of neurological sequelae increases from 0.9% below 65 years to 9% above 75 years [81]. Type I lesions are related to cerebral micro- and macro-emboliations, which are directly proportional to aortic manipulation and cardiotomy suctions (Figure 7.26) [21,22,36].
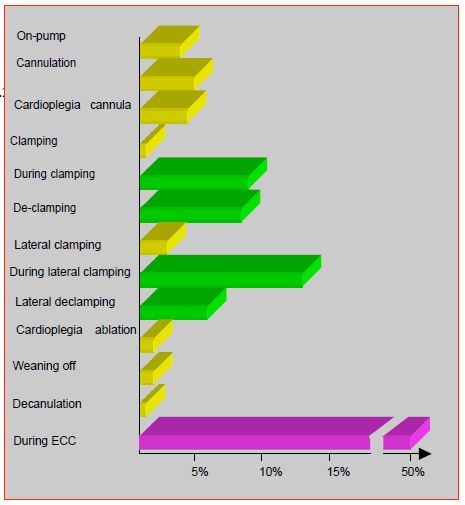
Figure 7.26: Frequency of cerebral embolisms diagnosed by transcranial Doppler during ECT. It can be seen that half occur during the various manipulations of the aorta. The other half are randomly distributed throughout the pump, without being associated with any surgical event [74].
- Half of the emboli detected by transcranial Doppler occur during instrumental manoeuvres on the aorta [74].
- The longer the ECC , the more emboli occur; this is probably related to the "sanding" effect of the aortic cannula jet against the atheromatous wall of the ascending aorta [14].
- The neurological status is influenced by the amount of blood aspirated by the cardiotomy suction and retransferred to the patient without prior cell saving procedure.
However, a clear relationship between the size of these emboli on transcranial Doppler and neurological deterioration has never been demonstrated. They are up to 6 times less numerous in beating heart surgery, without significantly altering postoperative neurological disorders, except in patients with a history of stroke who have a better prognosis with OPCAB [1,7,45,47].
Type II lesions are secondary to several phenomena: global cerebral hypoperfusion, anaemia, microembolisation, cerebral oedema, hyperthermia and inflammatory reaction [33]. Cognitive dysfunction is determined more by the composition of microemboli than by their number, because of the variations in the intensity of the inflammatory reaction induced locally according to the nature of the embolism (air, lipid, microparticle, etc) [36]. But these cognitive alterations are also part of a slow psychic decline linked to age and cerebrovascular disease (vascular disease, diabetes); they are essentially linked to pre-existing pathology, often sub-clinical, and to post-traumatic stress syndrome, which is poorly tolerated by elderly or frail people [9,35].
ECC has often been singled out as the main culprit for neurological sequelae, because manipulations associated are responsible for some of the brain embolisation and cognitive dysfunction. However, the off-pump coronary artery bypass (OPCAB) experience has shown that the absence of bypass surgery does not reduce these complications to zero. It is expected that the absence of aortic clamping and cannula jet will decrease the incidence of atheromatous emboli: the total number of embolic events is indeed lowered on transcranial Doppler [12,47]. However, there was no significant difference in stroke rate in meta-analyses comparing cardiac surgery with and without bypass surgery [17,60]. A reduction in clinical strokes has only been found in non-randomised studies, in octogenarians, or in patients with atheromatous aorta [24,71,89]. The incidence of type I lesions during beating heart coronary surgery remains around 2% [18]. In cases of extensive atheromatosis of the ascending aorta, OPCAB avoids clamping the aorta and probably decreases the risk of embolic stroke, but only the complete absence of aortic manipulation, using fully arterial grafts, can really decrease the incidence of stroke [15].
Cognitive neuropsychological impairment is also not exclusively related to bypass surgery, since its absence does not reduce it: the incidence of type II lesions is unchanged in beating heart bypass surgery [42,48,82]. It is only reduced after OPCAB in patients at high neurological risk, but not in patients without specific risk factors [82,83].
Management of the ECC
Postoperative neurological status is dependent of on-pump perfusion pressure outside a middle zone (50-80 mmHg) within which MAP level makes no difference. For example, a randomised clinical trial has clearly demonstrated that neuro-cognitive outcomes are better at MAP 80-100 mmHg rather than 50-60 mmHg [31]. At low mean pressure and pH-stat, the rate of cerebral embolism tends to increase because a greater proportion of blood flow is directed to the brain due to hypercapnic vasodilation [77]. However, the maintenance of cerebral selfregulation up to 40 mmHg mean pressure in moderate hypothermia (28°) and normocapnia (alpha-stat) means that pressure and flow characteristics during bypass surgery have relatively little influence on patient outcome in patients without particular neurological risks [4]. Certain circumstances suppress selfregulation and make cerebral flow totally pressure-dependent: deep hypothermia (< 25°C), hours following a hypothermic total circulatory arrest, diabetes, and presence of previous stroke [72]. On the other hand, 20% of patients have impaired selfregulation in bypass surgery, particularly during rewarming, which makes their cerebral oxygenation pressure dependent [57]. In the elderly, stiffness of the vascular tree increases the pressure differential (pulse pressure = PAs - PAd), increases cerebral vascular stress and reduces the range of selfregulation (see Chapter 5 Ventriculo-arterial coupling). The value of pulse pressure is an independent predictor of stroke after cardiac surgery [8].
Although flow rate is generally given priority over pressure, it appears that high pressure (> 60 mmHg) with low flow rate (< 1.2 L/m2 ) is less neurologically damaging than low pressure (< 30 mmHg) with high flow rate (> 2 L/m2 ) [19]. Ideally, MAP should be between 50 and 90 mmHg, proportional to the patient's age (MAP = age). In any case, it should remain above the low value of the selfregulation zone. Unfortunately, it varies between 40 and 80 mmHg (mean 66 mmHg) depending on the individual and the circumstances [13,41]. Cerebral saturometry (ScO2 , see below) offers a possibility of access to this data and allows MAP to be adapted to individual's cerebral needs.
In addition to optimal management of O2 supply to the brain by matching blood pressure, blood flow and haematocrit, the anaesthetist can influence the incidence of neurological complications by the choice of drugs used, since halogens and dexmedetomidine have neuroprotective effects, unlike propofol [9]. Statins and steroids do not significantly reduce neurocognitive sequelae [44,87].
The way the ECC is managed has a modest influence on neurological outcome. Regulation of acid-base balance in alpha-stat mode, which preserves cerebral selfregulation (and hence some vasoconstriction) and maintains oxygen delivery at low perfusion pressure (MAP of 45 mmHg), has better results than pH-stat, which maintains vasodilation by relative hypercarbia and promotes embolisation [59,76]. Type II lesions are less frequent in alpha-stat mode (27% incidence) than in pH-stat mode (44% incidence) [53]. Cerebral blood flow is halved in alpha-stat at 28°, and neurological failure is also halved when the duration of ECC exceeds 90 minutes [74]. The pH-stat mode probably makes sense during cooling and rewarming of operated cases in deep hypothermia (< 20°) and complete circulatory arrest, because cerebral vasodilation then ensures better temperature homogeneity in the brain [3,38].
Hypothermia offers some protection as it decreases cerebral metabolism and the effects of systemic inflammatory response (see Chapter 24 Cerebral effects of hypothermia) [85]. A lower stroke rate has been demonstrated with hypothermia (1.5% at < 28°C) compared to normothermia (4.5% at > 35°C), but the limit of a possible protection offered by hypothermia is the predominance of embolic events in the genesis of neurological sequelae. Moreover, the influence of temperature on type II lesions remains highly controversial. The major problem arises with rewarming, because the brain becomes transiently hyperthermic (38-39°) [10]. This rebound effect is all the more pronounced the faster the rewarming; it profoundly worsens the susceptibility of neurons to ischaemia and increases the extent of focal lesions [40,53]. Neurological sequelae are proportional to the fall in jugular venous saturation during rewarming [20]. Type II neuropsychological changes decrease with slower rewarming [32]. The rate of rewarming should therefore not exceed 1°C per 5 minutes, nor should the artery-esophagus temperature gradient exceed 2-3°C [11]. The blood temperature should not exceed 37°C [70]. In clinical practice, brain temperature is measured by tympanic temperature or by nasopharyngeal temperature with a nasal probe being pressed on the ethmoidal cells.
Haematocrit is unrelated to postoperative neurological status in young adults, as long as the Hb remains above 50 g/L [84]. In the elderly, cognitive impairment after coronary artery bypass grafting is greater when the minimum Ht is 15-17% than when it is >25% [23]. In children, a comparison between low (21%) and high (28%) Ht in bypass shows that neurological and mental developmental scores are significantly higher in the latter case [39]. The use of miniaturised minimal extracorporeal circuits (MECC) reduces the amount of priming fluid, decreases blood contact with foreign surfaces and eliminates the venous reservoir (blood contact with air). As a result, haemodilution and inflammatory response are reduced and the incidence of cognitive impairment is lowered [9,70]. Some substances such as aprotinin or steroids have an inhibitory effect on leukocyte activation and kallikrein release and tend to decrease the incidence of neurological sequelae [26,51].
Considering the scale of the problem, all possible means are being sought to reduce the frequency and extent of neurological sequelae. But the situation is ambivalent. Indeed, if hypoperfusion is the main cause of neurological disorders, high flow and high pressure must be maintained on bypass, whereas if embolism is the main factor, blood flow to the brain must be reduced [74]. On the other hand, drugs and different techniques have little effect on neurological disorders, apart from the total absence of manipulation of the ascending aorta when it is highly atheromatous.
| Neurological sequelae |
|
There are two types of postoperative neurological injury:
- Type I: Stroke with neurological sequelae
- Type II: cognitive impairment, reversible within weeks or months
Incidence of stroke: from 1.6% after simple coronary artery bypass grafting, to 5% after AVR and up to 17% in case of combined carotid surgery. Incidence of reversible cognitive impairment: 20-50%.
The two main mechanisms are embolisation and hypoperfusion. Embolisation accounts for 80% of type I lesions in adults, whereas hypoperfusion is more frequently involved in children. The MAP required to limit neurological sequelae in patients at risk is 70-80 mmHg. Their damage is compounded by the systemic inflammatory response and ischaemia/reperfusion injury.
Neurological disorders are multifactorial in origin, but the weight of evidence tends to show that patient-related risk factors (atheromatosis, anamnesis of stroke, preoperative cognitive disorders) are more important than those related to the procedure (operation in ECC or beating heart, embolisms, aortic clamping, etc). Cognitive impairment after cardiac surgery is more related to age decline and cerebrovascular disease than to intraoperative events, except for manipulation of the ascending aorta when it is highly atheromatous.
|
Cerebral protection
The aim of brain protection is to reduce sources of injury such as embolism and hypoperfusion, and to increase tolerance to insults such as ischaemia and inflammation. Unfortunately, there is no magic formula for this. Apart from lowering the metabolism through hypothermia and maintaining a balanced perfusion, there is no clearly effective way to improve the neurological prognosis. However, there are a number of measures that tend to decrease risk factors and increase some safety margin. Cerebral protection therefore covers several areas (for more details see Chapter 24 Cerebral protection in cardiac surgery and Protection in circulatory arrest).
- Maintenance of cerebral perfusion pressure; MAP 60-80 mmHg [55]. In fact, only 20% of strokes (type I lesions) are related to decreased perfusion pressure in adults, the remainder being embolic lesions [65]. In contrast, embolic strokes are rarer in children and the majority of neurological sequelae are related to cerebral ischaemia, particularly in case of circulatory arrest [46]. MAP has a greater impact on neurocognitive disorders (type II lesions).
- Maintenance of systemic oxygenation; each 10 mmHg decrease in minimum recorded PaO2 increases the risk of stroke by 20% (OR 1.23); a nadir PaO2 < 80 mmHg is directly associated with neurological sequelae [28].
- Decrease in the rate of ECC-related embolism in various ways [1,70]:
- Avoid clamping the ascending aorta when it is atheromatous, modification of the cannulation site, all-arterial grafts;
- Very restrictive use of cardiotomy suction;
- Washing and filtering of the aspirated blood prior to retransfusion or reinjection into the ECC circuit;
- Use of filtration system in the ascending aorta (Embol-X™) [66];
- Decreased risk of gas embolism by continuous insufflation of CO2 into the pericardium to replace ambient air with a more blood-soluble gas (with no clear effect on neurological sequelae) [36];
- Careful debulking before weaning off bypass.
- Epiaortic ultrasound; in high-risk groups such as patients with arteriosclerotic vasculopathy or elderly patients, epiaortic ultrasound can identify dangerous lesions of the ascending aorta and modify the surgical technique accordingly (avoidance of the most emboligenic plaques, subclavian or femoral cannulation) and decrease the incidence of embolic events [54].
- Maintenance of minimal cerebral perfusion (continuous low anterograde flow) during a period of circulatory arrest [78].
- Low venous pressure, especially when cannulating the superior vena cava in isolation (measured through the side arm of the introducer or through the proximal lumen of the PVC catheter).
- Decrease in the duration of ECC and, if necessary, the period in total circulatory arrest.
- Moderate hypothermia (28-32°); slow homogeneous rewarming (1° / 5 min, maximum blood temperature: 37°C).
- Moderate haemodilution: maintenance of Ht ≥ 26%. Each 10 g/L drop in Hb and each bag of blood transfused increases the risk of stroke by 30% (OR 1.28-1.37) [6].
- Maintain normoglycaemia (blood glucose < 10 mmol/L), regulate acid-base balance in alpha-stat mode, avoid hypercalcaemia [70].
- Improved biocompatibility of circuits; membrane oxygenators, heparinised circuits, mini-circuits without air-blood interface [70].
- Pharmacological means to decrease systemic inflammatory reaction and the cerebral oedema it triggers: corticosteroids, high-dose aprotinin, complement inhibitors (pexelizumab); no clear evidence of effect.
During circulatory arrest in bypass surgery, several measures can be taken to limit brain damage (see Chapter 18 Brain protection) [78].
- Cerebral hypothermia: this allows the duration of circulatory arrest to be extended from 4 minutes (37°C) to 12 minutes (28°C) and 35 minutes (18°C) without risking major neurological sequelae [43,49]. The cooling must be homogeneous and slow (0.5°/min).
- Continuous selective cerebral perfusion (10-15 mL/kg/min).
- Moderate hypothermia (28-30°C) with continuous cerebral and visceral perfusion via right subclavian and femoral cannulations.
- Trendelenburg position.
- Normoglycaemia (reduction in sequelae).
- Deep anaesthesia (curarisation), halogens (preconditioning).
- Warm up evenly and slowly (1°/5 min) otherwise risk of cerebral hyperthermia (maximum blood temperature: 37°C).
- Unproofed: magnesium, thiopental, nimodipine, steroids, mannitol.
In summary, neurological sequelae (type I) are primarily related to the embolic load, whereas hypotension plays a more modest role in adults (one quarter of cases) and acts preferentially on cognitive disorders (type II). While bypass surgery certainly contributes to this, it is only one out of many causal factors, as demonstrated by comparison with beating heart surgery. Manipulation of an atheromatous ascending aorta is probably the most dangerous procedure. One reason why the results of follow-up studies are not very discriminating is that preoperative neurological deficits are poorly investigated and attrition curves for brain function in control populations often do not exist. Apart from the common sense recommendations to maintain a normal O2 intake, the evidence base for possible neuroprotective measures is very weak [36].
| Cerebral protection in ECC |
|
There are a few ways to limit the risk of neurological sequelae after heart surgery:
- Reduce the risk of macroembolism (atheroma) and microembolism (debris, air, lipids)
- Limit or avoid aortic clamps (beating heart, all-arterial bypass)
- Limiting the duration of bypass surgery and the duration of aortic clamping
- Ensure cerebral perfusion (MAP > 70 mmHg) and O transport2 (Ht > 26%)
- Controlling temperature (moderate hypothermia and slow warming)
- Maintain normoglycemia and alpha-stat acid-base control
- Use new ECC technologies (mini-ECC, biocompatible materials)
- Visualise the ascending aorta (epiaortic ultrasound) and monitor oxygenation
(ScO2 )
- Administer pharmacological neuroprotection (very relative effectiveness)
Patient-related risk factors (atheromatosis, history of stroke, pre-operative cognitive impairment) are more important than those related to the procedure (bypass surgery or beating heart surgery).
With the exception of manoeuvres responsible for atheromatous embolism such as manipulation of the ascending aorta, the measures studied so far (bypass circuits, pump flow, pH and blood sugar regulation, hypothermia, pharmacological protection, etc.) have not provided indisputable evidence of their effectiveness in preventing neurological disorders. Only multimodal management can guarantee the "best possible outcome" in the current state of our knowledge.
In the event of complete circulatory arrest, the only proven means of reducing neurological sequelae are three:
- Maintaining minimal cerebral blood flow
- Limiting the duration of circulatory arrest
- Cool the brain to 18-20°C
|
Brain monitoring
The major question is obviously that of diagnosis: how to assess when brain is at risk during ECC? Several techniques can be considered [30]: EEG, evoked potentials, BIS™, transcranial Doppler, jugular venous saturation and near infrared spectroscopy (NIRS).
The EEG only reflects global cortical activity. EEG alterations are visible when cerebral blood flow has been halved; the EEG is isoelectric for a blood flow of 15-20 mL/100g/min (normal value: 50 mL/100g/min). Focal ischaemia may escape this monitoring. The 16 or 20 channel system is cumbersome and difficult to interpret and is usually replaced by a Compressed Spectral Array (CSA) brain function monitor, which displays a spectral analysis of the waves and requires only four electrodes (see Figure 19.14). The EEG is most useful for determining the level of brain cooling prior to circulatory arrest; interruption of perfusion should occur 5-10 minutes after electrical silence is achieved [36].
Auditory evoked potentials (AEP); the auditory brainstem response reflects neural activity between the cochlear nucleus and inferior colliculus; it is not affected by anaesthetic agents but varies directly with temperature. It is an excellent mean of monitoring the degree of neuronal inhibition by hypothermia, but the system is complex and requires a technical specialist [64].
The Bispectral Index (BIS™) analyses 4 variables of a bipolar EEG trace (amplitude, frequency, composition and phase coherence); an algorithm transforms it into a number between 0 (deep sleep) and 100 (awake). This number describes the depth of anaesthesia, but not the neuronal functioning [61]. The response depends on the type of anaesthesia; with propofol or halogen, the BIS value adequately predicts the response to the surgical stimulus, but opiates show no dose-response relationship [68]. Hypothermia (28-30°C) is responsible for a drop in BIS value, even when the halogen concentration remains stable [37]. A value of > 80 is common during rewarming [2]. However, protocols based on BIS have not been shown to be superior to those based on the expiratory concentration of halogen in preventing intraoperative arousal; BIS does not alter the concentration of halogen or reduce the incidence of arousal during surgery [5]. Its effectiveness is therefore uncertain [80]. Clinical experience shows that BIS collapses (value 10-15) during episodes of brain injury or severe haemodynamic instability, but this concordance does not make it a warning sign as it occurs as a consequence of cardiocirculatory shock [29,86].
Transcranial Doppler (TCD) measures blood flow in a large artery, usually the middle cerebral artery, and allows flow to be calculated, but the equipment is cumbersome, unstable, and unreliable in about 25% of patients [30]. A Doppler only measures the velocity of the red blood cells, not their number; thus haemodilution, which accelerates the flow, can lead to the belief that the oxygen supply has increased, whereas it has decreased. Its use as a monitor assumes that the velocity of the flow actually reflects the total blood flow, that the vessel diameter does not change, and that the sensor remains absolutely stable. Variations in Hb, viscosity, temperature, PaCO2 , and anaesthetic agents interfere considerably with the measurement [27]. Emboli are easily detected by the technique, especially during aortic clamping; they appear as HITS (High-intensity transient signals) whose morphology gives a key to their origin (bubble, atheroma, etc) [62].
Jugular venous saturation (SjO2 ) is a function of cerebral O extraction2 and global metabolic activity. Its normal value is 60-75%. The critical value is around 50% [74]. A value < 40% is associated with ischaemic brain damage and neurological sequelae [69]. It increases in the case of hyperaemia, hypercapnia or arteriovenous fistula. It decreases for systemic reasons (arterial desaturation, hypocpania, acute anaemia, hypotension) or cerebral reasons (intracranial hypertension, hyperthermia, vasospasm). It can be useful to confirm the decrease in metabolic demand before circulatory arrest.
Near-infrared spectroscopy (NIRS) allows the local measurement of cerebral haemoglobin oxygen saturation (ScO2 ) by two sensors placed at the frontotemporal angle on either side of the skull (see Figure 19.6). The emitted laser wavelength (770 - 910 nm) penetrates the skull and is scattered by the brain substance where a specific part of the spectrum is absorbed by oxygenated haemoglobin (HbO2 ) and another by reduced haemoglobin. The values displayed are very close to the cerebral venous saturation (SjO2 ) because three quarters of the cerebral blood is in the venous network and because the device uses the non-pulsatile components of the spectrum; normal value varies between 60 and 75% [56]. ECC does not change the baseline value, but the ScO2 tends to fall by 5-6 points when patient is off pump [75]. However, ischaemia occurring in a region other than the frontal area is not monitored, as the device only measures SO2 in 4 cm3 of brain on each side; however, the technique does allow differentiation between the two hemispheres. The ScO2 rises in hyperoxia and hypothermia (decreased metabolism), but also in brain death [79]. In hypothermic circulatory arrest, the nadir is reached within 15-20 minutes. A drop to a value of 30% indicates cerebral ischaemia; if it lasts longer than 10 minutes, it is associated with cognitive dysfunction [88]. The threshold of irreversibility is not yet known [67]. Due to hypothermic vasoconstriction, ScO2 values are lower in alpha-stat than in pH-stat regulation [50]. There are currently insufficient studies to prove that maintaining ScO2 within its normal range (about 66%), or correcting it if it deviates from this range, has a definite impact on the risk of stroke or neurocognitive disorders [16,90]. In clinical practice, a fall of 20% from baseline is considered significant; it occurs in 60% of ECCs and requires a series of therapeutic measures [75]. As soon as the ScO2 drops, O2 supply to the brain should be improved (see Haemodynamics, Blood Pressure) [25,73,75,88].
- Increase in MAP;
- Increase FiO2 and Ht (transfusion if Ht too low);
- Adjustment of the pump flow rate;
- Normocapnia if PaCO2 was lowered;
- Decreased brain metabolism: hypothermia, anaesthetic agents, curarisation;
- Repositioning of the head (unilateral change);
- Repositioning of venous cannulae or the heart in the operating field.
In general, ScO2 correlates well with these measures, but this improvement does not appear to have a significant impact on postoperative neurological sequelae in responders [34]. This demonstrates that ScO2 monitors not only cerebral oxygenation, but also O2 transport to all organs. Indeed, brain is the only organ that is easily accessible to a saturation sensor. As the brain has an auto-regulation system to maintain its O2 supply, a drop in its arteriovenous saturation indicates a greater decrease in O2 transport in the rest of the body. It is therefore not surprising that ScO2 correlates with postoperative haemodynamic dysfunction [58].
| Neurological monitoring |
|
EEG, transcranial Doppler and brain saturometry (ScO2 ) are the most reliable monitoring techniques. BIS is not a neurological monitoring technique.
The ScO2 has the best efficiency/complexity ratio:
- Normal value: 60 - 75%.
- Neurological risk threshold: 20% decrease from baseline, or value ≤ 40%.
- Rapid fall and duration of > 10 minutes to < 50% correlates with neurological status
- A drop in ScO2 should prompt immediate action to improve O2
to the brain
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- ABU-OMAR Y, BALACUMARASWAMI L, PIGOTT DW, et al. Solid and gazeous cerebral micro-embolization during off-pump, on-pump and open cardiac surgery procedures. J Thorac Cardiovasc Surg 2004; 127:1759-65
- ANDROPOULOS DB, STAYER SA, DIAZ LK, RAMAMOORTHY C. Neurological monitoring for congenital heart surgery. Anesth Analg 2004; 99:1365-75
- AOKI M, NORMURA F, STROMSKI ME, et al. Effects of pH on brain energetics after hypothermic circulatory arrest. Ann Thorac Surg 1993; 55:1093-1103
- ARROWSMITH JE, GROCOTT HP, REVES JG, et al. Central nervous system complications of cardiac surgery. Br J Anaesth 2000; 84:378-93
- AVIDAN MS, JACOBSOHN E, GLICK D, et al. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med 2011; 365:591-600
- BAHRAINWALA ZS, GREGA MA, HOGUE CW, et al. Intraoperative haemoglobin levels and transfusion independently predict stroke after cardiac operations. Ann Thorac Surg 2011; 91:1113-9
- BAR-YOSEF S, ANDERS M, MACKENSEN GB, et al. Aortic atheroma burden and cognitive dysfunction after coronary artery bypass graft surgery. Ann Thorac Surg 2004; 78:1556-62
- BENJO A, THOMPSON RE, FINE D, et al. Pulse pressure is an age-independent predictor of stroke development after cardiac surgery. Hypertension 2007; 50:630-5
- BHAMIDIPATI D, GOLDHAMMER JE, SPERLING MR, et al. Cognitive outcomes after coronary artery bypass grafting. J Cardiothorac Vasc Anesth 2017; 31:707-18
- BISSONNETTE B, HOLTBY HM, PUA DAJ, ET AL. Cerebral hyperthermia in children after cardiopulmonary bypass. Anesthesiology 2000; 93:611-8
- BORGER MA, RAO V. Temperature management during cardiopulmonary bypass: Effect of rewarming rate on cognitive dysfunction. Semin Cardiothorac Vasc Anesth 2002; 6:17-20
- Bowles BJ, Lee JD, Dang CR, et al. Coronary artery bypass performed without the use of cardiopulmonary bypass is associated with reduced cerebral microemboli and improved clinical results. Chest 2001; 119: 25-30
- BRADY K, JOSHI B, ZWEIFEL C, et al. Real-time continuous monitoring of cerebral blood flow selfregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010; 41:1951-6
- BROWN WR, MOODY DM, CHALLA VR, et al. Longer duration of cardiopulmonary bypass is associated with greater numbers of cerebral microemboli. Stroke 2000; 31:707-13
- Calafiore AM, Di Mauro M, Teodori G, et al. Impact of aortic manipulation on incidence of cerebrovascular accidents after surgical myocardial revascularization. Ann Thorac Surg 2002; 73: 1387-93
- CHAN MJ, CHUNG T, GLASSFORD NJ, et al. Near-infrared spectroscopy in adult cardiac surgery patients: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2017; 31:1155-65
- Cheng DC, Bainbridge D, Martin JE, Novick RJ. Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology 2005; 102:188-203
- CLEVELAND JC, SHROYER AL, CHEN AY, et al. Off-pump coronary artery bypass grafting decreases risk-adjusted mortality and morbidity. Ann Thorac Surg 2001; 72:1282-8
- COOK DJ. Neurologic effects. In: GRAVLEE GP (ed). Cardiopulmonary bypass. 2nd edition. Philadelphia, Lippincott, Williams & Wilkins, 2000, pp 403-31
- CROUGHWELL ND, NEWMAN NF, BLUMENTHAL JA, et al. Jugular bulb saturation and cognitive dysfunction after cardiopulmonary bypass. Ann Thorac Surg 1994; 58:1702-8
- DAVILA-ROMAN VG, BARZILAI B, WAREING TH, et al. Atherosclerosis of the ascending aorta. Stroke 1994; 25:2010-16
- DAVILA-ROMAN VG, BARZILAI B, WAREING TH, et al. Intraoperative ultrasonographic evaluation of the ascending aorta in 100 consecutive patients undergoing cardiac surgery. Circulation 1991; 84(Suppl III):47-53
- DE FOE GR, ROSS CS, OLMSTEAD EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Ann Thorac Surg 2001; 71:769-76
- Demaria RG, Carrier M, Fortier S, et al. Reduced mortality and strokes with off-pump artery bypass grafting surgery in octogenarians. Circulation 2002; 106(suppl I):I-5-10
- DENAULT A, DESCHAMPS A, MURKIN JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth 2007; 11:274-81
- DIELMAN JM, NIERICH AP, ROSSEEL PM, et al. Intraoperative high-dose dexamethasone in cardiac surgery:a randomized controlled trial. JAMA 2012; 308:1761-7
- DOBLAR DD. Cerebrovascular assessment of the high-risk patient: The role of transcranial Doppler ultrasound. J Cardiothorac Vasc Anesth 1996; 10:3-14
- DUNHAM AM, GREGA MA, BROWN CH, et al. Perioperative low arterial oxygenation is associated with increased stroke risk in cardiac surgery. Anesth Analg 2017; 125:38-43
- ENGLAND MR. The changes in bispectral index during a hypovolemic cardiac arrest. Anesthesiology 1999; 91:1947-9
- FEDOROW C, GROCOTT H. Cerebral monitoring to optimize outcomes after cardiac surgery. Curr Opin Anaesthesiol 2010; 23:89-94
- GOLD JP, CHARLESON MD, WILLIAMS-RUSSO P, et al. Improvement in outcomes after CABG. A randomized trial comparing intraoperative high versus mean arterial pressure. J Thorac Cardiovasc Surg 1995; 110:1302-14
- GRIGORE AM, GROCOTT HP, MATHEW JP, et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg 2002; 94:4-10
- GROCOTT HP, HOMI HM, PUSKAS F. Cognitive dysfunction after cardiac surgery: revisiting etiology. Semin Cardiothorac vasc Anesth 2005; 9:123-9
- HERINGLAKE M, GARBERS C, KÄBLER JH, et al. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology 2010; 114:58-69
- HO PM, ARCINIEGAS DB, GRIGSBY J, et al. Predictors of cognitive decline following coronary artery bypass graft surgery. Ann Thorac Surg 2004; 77:597-603
- HOGUE CW, PALIN CA, ARROWSMITH JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg 2006; 103:21-37
- HONAN D, DOHERTY D, FRIZELLE H. A comparison of the effects on bispectral index of mild vs moderate hypothermia during cardiopulmonary bypass. Eur J Anaesthesiol 2006; 14:1-6
- JONAS RA. Hypothermia, circulatory arrest, and the pediatric brain. J Cardiothorac Vasc Anesth 1996; 10:66-74
- JONAS RA, WYPIJ D, ROTH SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg 2003; 126:1765-74
- JONES T, ROY RC. Should patients be normothermic in the immediate postoperative period? Ann Thorac Surg 1999; 68:1454-5
- JOSHI B, ONO M, BROWN C, et al. Predicting the limits of cerebral selfregulation during cardioplumonary bypass. Anesth Analg 2012; 114:503-10
- KENNEDY ED, CHOY KC, ALSTON RP, et al. Cognitive outcomes after on- and off-pump coronary artery bypass grafting surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:253-65
- Kern FH, Ungerleider RM, Reves JG, et al. The effect of altering pump flow rate on cerebral blood flow and cerebral metabolism in neonates, infants and children. Ann Thorac Surg 1993; 58:1366-72
- KOMATSU R, YILMAZ HO, YOU J, et al. Lack of association between preoperative statin use and respiratory and neurologic complications after cardiac surgery. Anesthesiology 2017; 126:799-809
- LIU YH, WANG DX, LI LH, et al. The effects of cardiopulmonary bypass on the number of cerebral microemboli and the incidence of cognitive dysfunction after coronary artery bypass surgery. Anesth Analg 2009; 109:1013-22
- LOZANO S, MOSSAD E. Cerebral function monitors during pediatric cardiac surgery: can they make a difference? J Cardiothorac Vasc Anesth 2004; 18:645-56
- LUND C, HOL PK, LUNDBLAD R, et al. Comparison of cerebral embolization during off-pump and on-pump coronary artery bypass surgery. Ann Thorac Surg 2003; 76:765-70
- MARASCO SF, SHARWOOD LN, ABRAMSON MJ. No improvement in neurocognitive outcomes after off-pump versus on-pump coronary revascularisation: A meta-analysis. Eur J Cardiothorac Surg 2008; 33:961-70
- McCULLOUGH JN, ZHANG N, REICH DL, et al. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg 1999; 67:1895-931
- MORIMOTO Y, NIIDA Y, HISANO K, et al. Changes in cerebral oxygenation in children undergoing surgical repair of ventricular septal defects. Anaesthesia 2003; 58:77-83
- MURKIN JM. Adverse central nervous system outcomes after cardiopulmonary bypass. A beneficial effect of aprotinin? Semin Cardiothorac Vasc Anesth 2001; 5:282-5
- MURKIN JM. Neurocognitive outcomes: the year in review. Curr Opin Anesthesiol 2005; 18:57-62
- MURKIN JM, MARTZKE JS, BUCHAN AM, et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. II Neurologic and cognitive outcomes. J Cardiothorac Vasc Surg 1995; 110:349-55
- MURKIN JM, MENKIS AH, DOWNEY D, et al. Epiaortic scanning decreases cerebral emboli during aortic cannulation and application of partial occlusion clamp. Ann Thorac Surg 1999; 68:1461-7
- MURPHY GS, HESSEL EA, GROOM RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg 2009; 108:1394-417
- NOLLERT G, SHINOKA T, JONAS RA. Near-infrared spectrophotometry of the brain in cardiovascular surgery. J Thorac Cardiovasc Surg 1998; 46:167-75
- ONO M, JOSHI B, BRADY K, et al. Risks for impaired cerebral selfregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth 2012; 109:391-8
- PAQUET C, DESCHAMPS A, DENAULT AY, et al. Baseline regional cerebral oxygen saturation correlates with left ventricular systolic and diastolic function. J Cardiothorac Vasc Anesth 2008; 22:840-6
- PATEL RL, TURTLE MR, CHAMBERS DJ, et al. Alpha-stat acid-base regulation during cardiopulmonary bypass improves neuropsychologic outcomes in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 1996; 111:1267-79
- RAJA SG, Dreyfus GD. Off-pump coronary artery bypass surgery: To do or not to do? Current best available evidence. J Cardiothorac Vasc Anesth 2004; 18:486-505
- RAMPIL IJ. A primer for EEG signal processing in anesthesia. Anesthesiology 1998: 89:980-1002
- RINGELSTEIN EB, DROSTE DW, BABIKIAN VL, et al. Consensus on microembolus detection by TCD. Stroke 1998; 29:725-9
- ROACH GW, KANCHUGER M, MORA MANGANO C, et al. Adverse cerebral outcomes after coronary bypass surgery. N Engl J Med 1996; 335:1857-64
- RODRIGUEZ RA, EDMONDS HL, AUDEN SM, et al. Auditory brainstem-evoked responses and temperature monitoring during pediatric cardiopulmonary bypass. Can J Anaesth 1999; 46:832-9
- SALAZAR JD, WITYK RJ, GREGA MA, et al. Stroke after cardiac surgery: short- and long-term outcomes. Ann Thorac Surg 2001; 72:1195-201
- SCHMITZ C, WEINREICH S, WHITE J, et al. Can particulate extraction from the ascending aorta reduce neurologic injury in cardiac surgery? J Thorac Cardiovasc Surg 2003; 126:1829-38
- SCHWARTZ G, LITSCHER G. Transcranila cerebral oxymetry, transcranial Doppler sonography, and heart rate variability: useful neuromotitoring tools in anaesthesia and intensive care? Eur J Anaesthesiol 2002; 19:543-9
- SEBEL PS, LANG E, RAMPIL IJ, et al. A multicenter study of bispectral electroencephylogram analysis for monitoring anesthetic depth. Anesth Analg 1997; 84:891-9
- SHAABAN T, HARMER M, LATTO P. Jugular bulb oximetry during cardiac surgery. Anaesthesia 2001; 56:24-37
- SHANN KG, LIKOSKY DS, MURKIN JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg 2006; 132:283-90
- Sharony R, Bizekis CS, Kanchuger M, et al. Off-pump coronary artery bypass grafting reduces mortality and stroke in patients with atheromatous aortas: A case control study. Circulation 2003; 108 (suppl II):15-20
- SHROYER AL, COOMBS LP, PETERSON ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg 2003; 75:1856-64
- SLATER JP, GUARINO T, STACK J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg 2009; 87:36-44
- STUMP DA, JONES TJJ, RORIE KD. Neurophysiologic monitoring and outcomes in cardiovascular surgery. J Cardiothorac Vasc Anesth 1999; 13:600-13
- SUBRAMANIAN B, NYMAN C, FRITOCK M, et al. A multicenter pilot study assessing regional cerebral oxygen desaturation frequency during cardiopulmonary bypass and responsiveness to an intervention algorithm. Anesth Analg 2016; 122:1786-93
- SUNGURTEKIN H, BOSTON US, COOK DJ. Bypass flow, mean arterial pressure, and cerebral perfusion during cardiopulmonary bypass in dogs. J Cardiothorac Vasc Anesth 2000; 14:25-8
- SUNGURTEKIN H, PLÖCHL W, COOK DJ. Relationship between cardiopulmonary bypass flow rate and cerebral embolization in dogs. Anesthesiology 1999; 91:1387-93
- SVYATETS M, TOLANI K, ZHANG M, et al. Perioperative management of deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2010; 24:644-55
- TAILEFER MC, DENAULT Y. Cerebral near-infrared spectroscopy in adult heart surgery: systematic review of its clinical efficacy. Can J Anesth 2005; 52:79-87
- TIREN C, ANDERSON RE, BARR G, et al. Clinical comparison of three different anaesthetic depth monitors during cardiopulmonary bypass. Anaesthesia 2005; 60:189-93
- TUMAN KJ, McCARTHY RJ, NAJAFI RJ, IVANKOVICH AD. Differential effects of advanced age on neurologic and cardiac risks of coronary operations. J Thorac Cardiovasc Surg 1999; 104:1510-7
- Van Dijk D, Jansen EW, Hijman R, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA 2002; 287: 1405-12
- VAN DIJK D, SPOOR M, HIJMAN R, et al. Cognitive and cardiac outcomes 5 years after off-pump vs on-pump coronary artery bypass graft surgery. JAMA 2007; 297:701-8
- VAN WERMESKERKEN GK, LARDENOYE JWH, HILL SE, et al. Intraoperative physiologic variables and outcome in cardiac surgery. Part II. Neurologic outcome. Ann Thorac Surg 2000; 69:1077-83
- WARREN OJ, SMITH AJ, ALEXIOU C, et al. The inflammatory response to cardiopulmonary bypass: Part 1 - Mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009; 23: 223-31
- WELSBY IJ, RYAN JM, BOOTH JV, et al. The bispectral index in the diagnosis of perioperative stroke: A case report and discussion. Anesth Analg 2003; 96:435-7
- WHITLOCK RP, DEVEREAUX PJ, TEOH KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 26:1243-53
- YAO FSF, TSENG CCA, HO CYA, et al. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2004; 18:553-8
- ZANGRILLO A, Crescenzi G, Landoni G, et al. Off-pump coronary artery bypass grafting reduces postoperative neurologic complications. J Cardiothorac Vasc Anesth 2005; 19:193-6
- ZHENG F, SHEINBERG R, YEE MS, et al. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg 2013; 116:663-76