- Organic or primary MI: the structure of the mitral leaflets and annulus is pathological, as in myxoid degeneration or rheumatic fever;
- Functional or secondary MI: the mitral valve apparatus is normal but the LV is diseased, as in ischaemic alteration of parietal kinetics or ventricular dilatation.
The extent of MI depends on the circulatory conditions. In general, this dependence is more pronounced in functional MI than in organic MI. Furthermore, the variability of MI as a function of haemodynamics is not identical in the two groups (see Physiopathology, MI and haemodynamics). It is therefore important for the anaesthetist to understand the aetiology and function of MI in order to ensure the patient's circulatory stability. In addition, it is essential for the anaesthetist-echocardiographer to be able to elucidate the mechanism of MI, as the management and surgical treatment will be very different depending on the type of pathology [17].
The aetiopathogenesis of MI is diverse, and in Europe and North America it is distributed as follows [4,11,13,18,24]
- Degenerative disease (45% of cases);
- Ischaemic or dilatative cardiomyopathy (27% of cases, increasing);
- Bacterial endocarditis (13% of cases);
- Rheumatic fever (12% of cases, decreasing);
- Congenital diseases (3% of cases).
Rheumatic fever is much more common in non-industrialised countries.
Degenerative diseases
Degenerative diseases are the most common, with a prevalence of 2% of the population in Europe [13]. It accounts for two thirds of the cases operated on. There are two anatomopathological types [5,19].
- Myxoid degeneration consisting of extensive deposits of mucopolysaccharides in the matrix (Barlow's disease). The leaflets and chords are thickened, gelatinous and yellowish; excess tissue renders the leaflets redundant, elongated and swollen; the annulus is dilated. Lesions may affect other valves: tricuspid (30%), aortic (10%) or pulmonary (2%). The disease progresses slowly.
- Fibro-elastic degeneration, characterised by a lack of collagen and elastin, which makes the layers and chords translucent and thin, but not redundant; it is typical of old age. It develops more rapidly.
Barlow's disease (see Barlow's disease) is a fairly broad condition, ranging from a benign syndrome with a female predominance to a severe structural disease of the mitral valve with severe insufficiency [6]. In the West, Barlow's disease is very common, affecting 1.7-2.4% of the adult population [15]. However, it is associated with significant MI in only 10-15% of cases. Severe insufficiency develops most commonly in men, usually after the age of 50, and leads to surgical repair in only 5% of patients [3,27]. Valve lesions consist of thickened, redundant leaflets (> 3 mm) in which collagen is partially replaced by mucopolysaccharide deposits, resulting in myxoid degeneration that appears yellowish in the surgical field. Typically, the anterior leaflet measures ≥ 35 mm in length and ≥ 3 mm in thickness (instead of 25 mm and 0.9 mm, respectively). The valve becomes inadequate and one or both leaflets prolapse and become inflated. The chords, whose collagen is also affected, stretch under excessive traction and some eventually rupture. Isolated chord tears may also occur. In 65% of cases, the lesion is located on the middle festoon of the posterior leaflet (P2).
Video: Three-dimensional view from the LA of a prolapse of the middle festoon of the posterior leaflet (P2), located in the lower middle of the valve on the on-screen image.
Echocardiography shows that degenerative disease can take several forms (Figure 11.64).
- Ballooning of a leaflet; part of the body of a leaflet protrudes into the LA, but the coaptation point is in the normal position on the ventricular side of the plane of the mitral annulus. Ballooning is associated with myxoid degeneration or rupture of a 2nd order chord (implanted on the body of the leaflet).
- Flaccid valve and excess tissue.
Video: Extended mitral prolapse with ballooning and remodelling of both leaflets, and chord ruture on the posterior leaflet (left on screen), in a 90° 2-cavity view.
- Prolapse; the point of coaptation is > 2 mm posterior to the plane of the mitral annulus (long-axis view of LV 120°).
Video: the coaptation point recedes behind the plane of the mitral annulus; the two leaflets do not coapt during systole.
Video: Prolapse of the middle festoon of the posterior leaflet (P2, on the right of the screen) in a 4-cavity 0° view.
Video: Prolapse of the middle festoon of the posterior leaflet (P2, on the right of the screen) in a bicommissural view (here at 90°); the prolapse appears above the medial part of the anterior leaflet.
Video: Prolapse of the middle festoon of the posterior leaflet (P2), visible on the left of the screen in this 120° long-axis view of the LV; the leaflet is reshaped and the regurgitant orifice is large; in the centre of the screen, truncated view of the aortic valve.
- Flail leaflet associated with elongation or rupture of a first-order chord; chord length measured in transgastric view 90-120°. MI is meso-tele-systolic in elongations but holo-systolic in ruptures.
Video: P2 prolapse with chord rupture on an enlarged and redundant posterior leaflet; degenerative lesions on both leaflets (130° long-axis view).
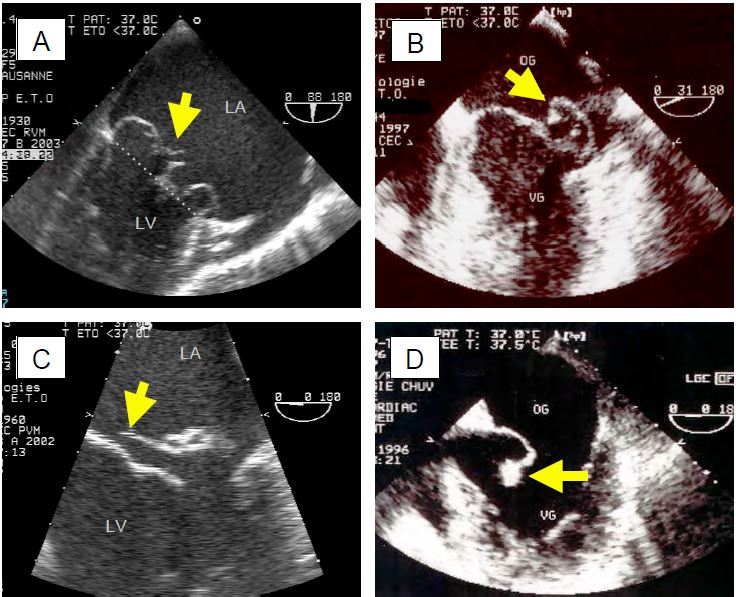
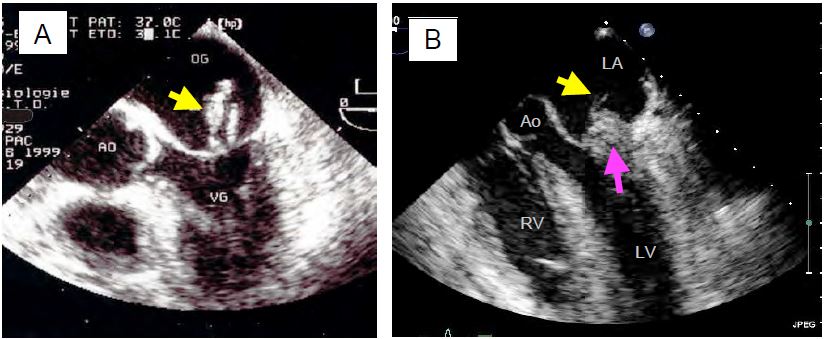
Figure 11.64: TEE images of mitral regurgitation due to myxoid degeneration. A: Excess tissue with ballooning, local thickening and prolapse of both leaflets; the arrow indicates displacement of the leaflet tips behind the plane of the mitral annulus (dotted line). B: Isolated prolapse of P2. C: P2 prolapse with cord rupture (indicated by the arrow). D: Complete rupture of the papillary muscle with a fragment of the column attached to the end of the anterior leaflet.
Prolapse most commonly affects the middle festoon of the posterior leaflet (P2, 57% of cases) and the middle part of the anterior leaflet (A2, 33% of cases); the posterior part of the posterior leaflet is affected in 28% of cases [20].
Acute rheumatic fever
Acute rheumatic fever (ARF) has become a rare cause of MI in developed countries (12% of MIs), but is still common in developing and emerging countries [13]. MI may occur during the acute phase of ARF in association with myocarditis or pancarditis, in which case it is due to annular and ventricular dilatation. Subsequently, as the disease progresses, there is significant thickening and retraction of the leaflets; they become rigid and have little mobility; the chords become fibrous, thickened and shortened. Commissural fusion results in mitral disease in which stenosis usually predominates over regurgitation.
Video: Mitral disease in 120° long-axis view; presence of mitral insufficiency in systole (central jet of regurgitation) and accelerated flow through the stenosis in diastole (zone of concentric acceleration on the atrial side and swirling flow with aliasing through the valve).
Ischaemic mitral insufficiency
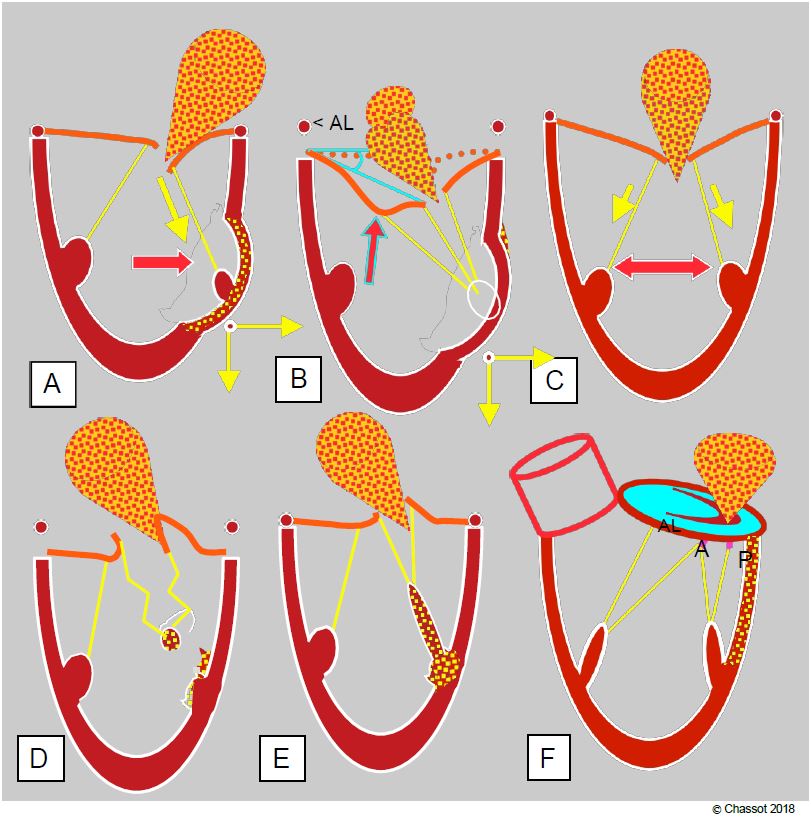
Mitral insufficiency occurs in 10-30% of coronary artery operations [5]. Left untreated, it significantly worsens the prognosis of coronary artery bypass grafting, but its repair is not trivial: the mortality of coronary revascularisation combined with mitral repair for severe MI is 3-7%, compared with an average of 1-2% for simple revascularisation [10,12]. This is due to several mechanisms (Figure 11.65).
- Akinesia or dyskinesia of the ventricular wall resulting in lateral and/or apical displacement of a papillary muscle in systole; the traction applied to the corresponding chords prevents the leaflet from reaching its coaptation point in systole (systolic motion restriction).
Video: Severe mitral insufficiency due to anterior akinesia after coronary artery bypass grafting.
- Ischaemic cardiomyopathy with dilatation and global hypokinesia of the LV. Enlargement of the ventricular cavity in systole does not allow the chords to move sufficiently to allow the leaflets to meet at the coaptation plane; the leaflets are held below the coaptation plane and the valve leaks.
- Pillar rupture; partial rupture results in tilting of the corresponding commissure into the LA during systole. Total abutment rupture causes such massive MI and profound cardiogenic shock that 50-75% of patients die before reaching the operating theatre. Rupture occurs in three quarters of cases in the posterior abutment because its vascularisation by the RCA alone is more fragile than that of the anterior abutment, which is vascularised by the IVA and CX.
Video: Tilt of the posterior commissure due to partial rupture of the posterior papillary muscle.
Video: Total rupture of the anterior papillary muscle, with a fragment dangling at the end of the chords of the anterior leaflet; this lesion leads to cardiogenic shock, which is often lethal because the mitral valve is no longer functional.
- Papillary ischaemia: non-contraction of a papillary muscle in systole results in excessive length of the chords; the point of coaptation of the leaflets is then inverted into the LA. When superimposed on parietal akinesia, papillary ischaemia partially corrects systolic mitral leaflet retraction [21].
- Akinesia of the basal wall: The non-contraction of the mitral annulus in systole does not allow the leaflets to coapt over a sufficient surface area for the valve to be leaktight; MI occurs by annular dilatation. This dilation occurs mainly in the posterior part of the annulus, as the anterior part is attached to the fibrous trine.
Like all secondary insufficiencies, ischaemic MI is very sensitive to hyperdynamic conditions: it increases during exercise or sympathetic stimulation and decreases during general anaesthesia or arterial vasodilatation [19]. This may lead to a significant underestimation of MI in the operating theatre compared with the cardiology office [26].
The first two categories account for 76% of cases and are a good indication for mitral annuloplasty if the MI is severe, whereas ischaemia and papillary rupture are more likely to be indications for valve replacement [16]. Unfortunately, the recurrence rate after simple annuloplasty for ischaemic MI is about 30% at 1 year [2]. As the pathology is in the underlying ventricle and not in the valve, interventions on the subvalvular apparatus may be indicated: repositioning of the papillary muscles, revascularisation, resynchronisation, cutting of the secondary chords (Figure 11.65B) [19]. In any case, the treatment of ischaemia includes coronary revascularisation.
Figure 11.65: Ischaemic mitral insufficiency. MI may be restrictive type IIIb (A, B and C), prolapsed type II (D and E) or functional type I (F) (see pathophysiology). A: Segmental akinesia or dyskinesia driving the column outwards and towards the apex in systole; the corresponding leaflet is restrictive; the MI is eccentric. B: Segmental akinesia or dyskinesia driving the column outwards with excessive traction on the 2nd chords; this results in a gull-wing image of the anterior leaflet. < AL: angle of the anterior leaflet at its tip with the plane of the annulus in systole. Surgical excision of the 2nd chords may be curative in this case. C: Ischaemic cardiomyopathy with homogeneous dilatation of the LV; restrictive MI is central. D: Partial or complete rupture of a column causing total tilting of the commissure in the LA during systole; a piece of the ruptured column is seen oscillating at the end of the chords. E: Ischaemia of a pillar causing prolapse of the corresponding commissure, because the pillar stretches instead of contracting during systole. F: Basal ischaemia causing posterior dilatation of the mitral annulus.
Restrictive mitral insufficiency
MI is said to be restrictive when excessive traction on the chords due to ventricular dilatation (LVOT > 4.0 cm) maintains the coaptation point below the plane of the mitral annulus and prevents the leaflets from joining during systole. This MI is functional and follows changes in LV size according to haemodynamic conditions. Its monitoring during surgery is an excellent marker of the degree of ventricular dysfunction (see Pathophysiology). Although the mitral leaflets increase in size by up to 35% to compensate for this coaptation defect [9], a vicious circle develops between LV dilatation, which limits valve closure, and regurgitation, which causes LV volume overload and further increases LV dilatation.
Video: Severe restrictive mitral insufficiency due to dilatation and failure of the LV.
Bien que la taille des feuillets mitraux augmente jusqu'à 35% pour compenser ce défaut de coaptation [9], il s'installe un cercle vicieux entre la dilatation du VG qui limite la fermeture de la valve et la régurgitation qui provoque une surcharge de volume du VG et qui augmente encore sa dilatation.
Endocarditis
Bacterial endocarditis affects an abnormal valve, even if the pathology is benign. It leads to vegetations, perforations, leaflet tears or abscesses (Figure 11.66) [4,25]. Vegetations are always located on the upstream (atrial) side of the leaflets. Mitral endocarditis is less common than aortic endocarditis, with which it may be associated. Despite advances in cardiac surgery and infectious diseases, the 1-year mortality rate is still around 30% [7].
Video: Vegetation attached to the posterior mitral leaflet and floating in the LA.
Video: Endocardial abscess at the base of the anterior leaflet.
Video: Colour flow showing mitral perforation in the same case as the previous video.
The vegetations are always located on the upstream (atrial) side of the leaflets. Mitral endocarditis is rarer than aortic endocarditis, with which it may be associated. Despite advances in cardiac surgery and infectious diseases, the 1-year mortality rate is still around 30% [7].
Figure 11.66: TEE images of mitral endocarditis. A: Large, elongated vegetations; like all vegetations, they are located on the downstream side of the leaflets (atrial side). B: Massive vegetation invading the entire posterior leaflet (purple arrow) surmounted by small filiform vegetations (yellow arrow).
Other etiologies
With age, the mitral valve loses its tightness without being abnormal; it is common to find small regurgitations of no pathological or haemodynamic significance from the age of 60 on. Moderate to severe MI occurs in 6% of patients aged 65-75 years and 9% of those aged >75 years [22]. There are other causes of MI.
- Dynamic obstruction of the LV outflow tract with systolic anterior motion (SAM) of the mitral valve coaptation (see Dynamic subaortic stenosis);
- Rare degenerative tissue disorders: Ehler-Danlos syndrome, Marfan syndrome;
- Congenital conditions: parachute mitral valve, common atrio-ventricular canal cleft.
Mitral annular calcification
Mitral annular calcification (MAC) is a degenerative process consisting of calcific deposits and thickening of the mitral annulus (>4 mm in severe cases). It occurs in 10% of the population, with a predominance in women [8]. It is common in the elderly, patients with renal failure on haemodialysis, hypertensive patients, polyvascular patients and patients with calcified aortic stenosis. It most commonly occurs in the posterior leaflet and can progressively involve one third (moderate MAC) or more than half (severe MAC) of the mitral annulus [1]. Sometimes the entire posterior leaflet becomes immobile.
Video: Mitral insufficiency due to massive calcification of the mitral annulus and posterior leaflet (120° long-axis view); the MI is eccentric, directed towards the interatrial septum due to rigidity of the posterior leaflet.
Histologically, the process resembles calcification of atheromatous plaques. It is directly associated with cardiovascular risk and arrhythmias (HR 1.6-1.9) [14,23].
On echo, the lesion appears as highly echogenic, irregular and bumpy areas, producing large shadow cones distally. Because it prevents the annulus from contracting normally (annular area decreases by 20-25% in systole), it is often the cause of mild to moderate MI. It generally precludes mitral valve surgery, but prosthetic valve implantation is often associated with paravalvular leaks due to mismatch between the valve and the calcified annulus. Decalcification and surgical reconstruction of the annulus carry a high risk of tearing of the atrioventricular junction (cardiac rupture) and injury to the circumflex artery (lateral ischaemia) [1].
| Etiology of mitral insufficiency (MI) |
|
Organic MI: structural damage to the leaflets and chords: Functional MI: normal valve but ventricular damage: |
References
- ABRAMOWITZ Y, JILAIHAWI H, CHAKRAVARTY T, et al. Mitral annulus calcification. J Am Coll Cardiol 2015; 66:1934-41
- ACKER MA, BOLLING S, SHEMIN R, et al. Mitral valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014; 370:23-32
- AVIERINOS JF, INAMO J, GRIGIONI F, et al. Sex differences in morphology and outcomes of mitral valve prolapse. Ann Intern Med 2008; 149:787-95
- BIRMINGHAM G, RAHKO P, BALLENTYNE F. Improved detection of infective endocarditis with transesophageal echocardiography. Am Heart J 1992; 123:774-9.
- BONOW RO, BRAUNWALD E. Valvular heart disease. In: ZIPES DP, et al, eds. Braunwald's heart disease. A textbook of cardiovascular medicine. 7th edition. Philadelphia: Elsevier Saunders, 2005, 1553-632
- BOUDOULAS H, KOLIBASH, AJ, BAKER P, et al. Mitral valve prolapse and the mitral valve prolapse syndrome: A diagnostic classification and pathogenesis of symptoms. Am Heart J 1989; 118:796-803
- CAHILL TJ, BADDOUR LM, HABIB G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69:325-44
- CARPENTIER A, PELLERIN M, FUZELLIER JF, RELLAND JY. Extensive calcification of mitral valve annulus: Pathology and surgical management. J Thorac Cardiovasc Surg 1996; 111:718-29
- CHAPUT M, HANDSCHUMACHER MD, TOURNOUX F, et al. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008; 118:845-52
- ELLIS SG, WHITLOW PL, RAYMOND RE, SCHNEIDER JP. Impact of mitral regurgitation on long-term survival after percutaneous coronary intervention. Am J Cardiol 2002; 89:315-8.
- FENSTER MS, FELDMAN MD. Mitral regurgitation: an overview. Curr Probl Cardiol 1995; 20:193-228
- FERGUSON T, DZIUBAN F, EDWARDS F, et al. STS National Database: current changes and challenges for the new millenium. Ann Thorac Surg 2000; 69:680-91
- FOSTER E. Mitral regurugitation due to degenerative mitral-valve disease. N Engl J Med 2010; 363:156-65
- FOX CS, VASAN RS, PARISE H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation 2003; 107:1492-6
- FREED LA, LEVY D, LEVINE RA, et al. Prevalence and clinical outcome of mitral valve prolapse. N Engl J Med 1999; 341:1-7.
- GILLINOV AM, WIERUP PN, BLACKSTONE EH, et al. Is repair preferable to replacement for ischaemic mitral regurgitation? J Thorac Cardiovasc Surg 2001; 122:1125-41
- GISBERT A, SOULIERE V, DENAULT AY, et al. Dynamic quantitative echocardiographic evaluation of mitral regurgitation in the operating department. J Am Soc Echocardiogr 2006; 19:140-6
- LANCELLOTTI P, MOURA L, AGRICOLA E, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010; 11:307-32
- LEVINE RA, HAGÉGE AA, JUDGE DP, et al. Mitral valve diasease - morphology and mechanisms. Nat Rev Cardiol 2015; 12:689-710
- MASLOW A. Mitral valve repair: an echocardiographic review: Part I. J Cardiothorac Vasc Anesth 2015; 29:156-77
- MESSAS E, GUERRERO JL, HANDSCHUMACHER MD, et al. Paradoxic decrease in ischemic mitral regurgitation with papillary muscle dysfunction. Circulation 2001; 104:1952-7
- NKOMO VT, Gardin JM, SKELTON TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005-11
- O'NEAL WT, EFIRD JT, NAZARIAN S, et al. Mitral annular calcification and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis. Europace 2015; 17:358-63
- OTTO CM. Clinical practice. Evaluation and management of chronic mitral regurgitation. N Engl J Med 2001; 345:740-6
- SANFILIPPO AJ, PICARD MH, NEWELL JB, et al. Echocardiographic assessment of patients with infectious endocarditis: prediction of risk for complication. J Am Coll Cardiol 1991; 18:1191-9
- SANFILIPPO F, JOHNSON C, BELLAVIA D, et sl. Mitral regurgitation grading in the operating room: a systematic review and meta-analysisi comparing preoperative and intraoperative assessments during cardiac surgery. J Cardiothorac Vasc Anesth 2017; 31:1681-91
- WILCKEN DEL, HICKEY AJ, Lifetime risk for patients with mitral valve prolapse of developing severe valve regurgitation requiring surgery. Circulation 1988; 78:10-8