Coagulation factors
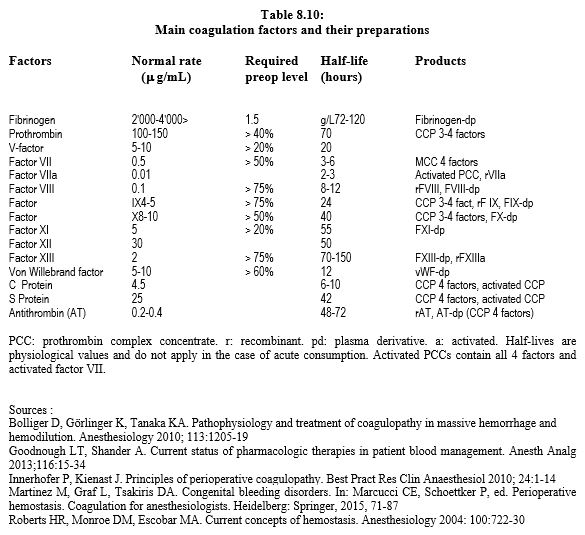
Many coagulation factors are available in isolated or combined form (Table 8.10). Their primary indication, accepted by health authorities in most countries, is treatment of congenital coagulopathies. Some have been approved to reverse the effect of anti-vitamin K agents (AVKs). However, their most common use is for their potential effect on consumption coagulopathies encountered in massive bleeding. In reality, this indication is often off-label. Most of these factors have some efficacy in reducing blood loss, but large controlled studies, where they exist, tend to show that this effect is rather modest, and that it comes along with a certain thrombotic risk, probably around 1-3% [11].
On the other hand, the cut-off value for factor administration is based on the norm for healthy individuals. It is only meaningful if the patient is bleeding and may vary according to bleeding risk and clinical context [1]. With the exception of congenital coagulopathies identified in patients already receiving factor replacement, prophylactic factor transfusion is not recommended before surgery.
Fresh frozen plasma (FFP)
Fresh frozen plasma is an economical source of clotting factors and a convenient way to maintain the oncotic power of plasma, although ABO grouping must be respected. Unfortunately, its indications are based only on data of low evidence and on studies, mostly observational, of modest quality of evidence [34,39]. The following indications are usually admitted.
- Reversal of AVKs in patients with intracranial haemorrhage or massive bleeding when prothrombin complex concentrates are not available; in haemorrhagic stroke, mortality is significantly reduced (OR 0.29) [27].
- Infusion component in case of plasma exchange (e.g. thrombocytopenic purpura).
- Replacement of a coagulation factor deficiency that does not exist in an isolated form (e.g. factor V or factor XII).
- Replacement of multiple coagulation factor deficiencies leading to massive blood loss, when factor testing and targeted replacement are not available.
- Prevention of haemodilution in polytransfused patients. This is only recommended in case of massive transfusion; in this situation it reduces mortality (OR 0.38) and the incidence of multi-organ failure (OR 0.40) [27]. There is no statistical argument for or against a fixed ratio of blood bags (1:1 to 1:3), but the latter is a practical benchmark in emergency situations [34].
Apart from massive transfusion, administration of FFP during haemorrhagic surgery, liver surgery or after ECC does not improve prognosis, but tends to increase mortality (OR 1.22-3.83) [27,33]. Because of their poor correlation with circulating levels of coagulation factors, preoperative tests (PT, INR, aPTT) have little relationship with surgical bleeding risk. Therefore, the indication for FFP based on decreased laboratory values is unfounded and has not been shown to have a significant effect on intraoperative bleeding or transfusion rates [21].
Because of the low concentration of factors in FFP (about 0.5 g of fibrinogen per vial), the amount to be infused to replenish a patient is considerable. The standard dose of 10-15 mL/kg is usually insufficient, and only a dose of 30 mL/kg is likely to normalise serum levels, at the cost of increasing the circulating volume by about 2 litres [6]. Prophylactic use is ineffective in compensating for surgical consumption coagulopathy and in reducing the number of blood transfusions, but carries a clear risk of volume overload and immunological complications [4] (see Chapter 28 Transfusion of Derived Products). Once frozen, FFP can be stored for a further 24 hours at 4°C; at room temperature it should be infused within half an hour [20].
There are four major complications associated with transfusion of FFP (see Chapter 28 Blood product risks) [21].
- Hypervolaemia and congestive haemodynamic failure (TACO, transfusion-associated circulatory overload) due to excess volume transfused; its incidence increases when ventricular dysfunction is present.
- Febrile reaction (common), infectious contamination (incidence 0.8‰).
- Allergic reaction (incidence 1-1.5%) with urticaria, hypotension and bronchospasm.
- Lung injury: TRALI (transfusion-related acute lung injury) triggered by donor plasma antibodies. The incidence of TRALI is proportional to the amount of plasma administered and ranges from 1:2,000 to 1:50 [21]. Regardless of the indication, FFP transfusion triples risk of pulmonary complications (OR 2.92) [27]. Given the high level of anti-HNA and anti-HLA antibodies in multiparous women, the use of male-only blood for FFP preparation has reduced the incidence of allergies and TRALI.
New preparations of FFPs are coming on to the market with some advantages [39].
- Octaplas® : purified preparation from 1,500 donors, virus and prion free; ABO grouping required.
- Uniplas® : variant in which anti-A and anti-B antibodies are extracted; no ABO grouping.
- LyoPlas® : freeze-dried plasma from a single donor, without blood cells, whose pathogens and viruses are destroyed by ultraviolet irradiation; advantage: conservation at room temperature for 15 months.
In summary, the accepted indications for FFP are massive transfusions, unavailability of isolated coagulation factors and plasma exchange [34]. In other situations, risks are likely to outweigh benefits.
Fibrinogen
Fibrinogen (factor I) is a large glycoprotein (340 kDa) that circulates in soluble form at a level of 2-4 g/L; this level increases to 6 g/L during trauma or surgery. In the absence of consumption, fibrinogen has a half-life of 100 hours. It has three main actions [26].
- In its soluble form, it acts as a linker between platelets by binding to GP IIb/IIIa receptors on platelets.
- When cleaved by thrombin (factor IIa), it is converted into fibrin monomers, which polymerise to form a firm, insoluble network; the viscoelastic firmness of the thrombus is directly correlated with the level of the substance: it is maximal at a level of 3.6 g/L [29]. The fibrin network only becomes firmly and irreversibly interconnected when Factor XIII has covalently bound to it (Figure 8.5).
- It functions as a coagulation inhibitor through its antithrombin I action.
Fibrinogen is the first element to decrease in blood loss, reaching a critical level of 1.5 g/L when loss of circulating volume is 50% [15]. Because of its key role in coagulation, administration of 2-8 gm at the onset of haemorrhage improves clot firmness and significantly reduces blood loss and transfusion. Several randomised studies show a clear reduction in the need for allologous blood bags, platelets and plasma in patients who have received fibrinogen [19,29]. The ZEPLAST trial in complex cardiac surgery shows that fibribogen supplementation reduces postoperative bleeding and transfusion compared to FFP administration [32]. However, some studies do not find a gain in transfusions but do show an improvement in clot firmness as measured by ROTEM™ (FIBTEM: MCF 14 mm) (see Intraoperative testing) [7,31]. Finally, others find no benefit to fibrinogen infusion, either at a fixed dose [16] or in relation to blood loss [28]. The level of 2 g/L seems to be the limit below which haemostasis is impaired, but there is no real threshold because rate of bleeding increases linearly and continuously with fall in preoperative fibrinogen level [18]. As a result, current recommendations have raised the desirable fibrinogen value for acute bleeding and consider a level of <2 g/L as hypofibrinogenemia [2,35]. The administration of 3 gm fibrinogen to a 70 kg patient increases the plasma level by approximately 1 g/L [26]. The most accurate management is based on FIBTEM results: 25-50 mg/kg are required to maintain CMF > 10 mm [40]. However, the question of prophylactic administration of fibrinogen before or after ECC in cardiac surgery patients with levels < 2.5 g/L is still open, especially since the determination of fibrinogen by the classical Clauss method is rather inaccurate.
Fibrinogen replacement can occur in three different ways [26].
- Fresh frozen plasma (FFP) contains about 2 g/L (0.4 g/U) of fibrinogen, which requires large amounts of plasma (30 mL/kg) to be given, with the risk of volume overload, immunological reaction and viral or bacterial contamination.
- Cryoprecipitate is a human plasma concentrate, one unit of which contains 388 mg of fibrinogen; risks of contamination and immunological reactions have led to its withdrawal from most of the European market.
- Lyophilised and pasteurised fibrinogen does not present these risks; the powder (1 g/U) once diluted, product concentration rises to 20 g/L, which avoids fluid overload. This is the most suitable form, except that it costs about €200 for 1 gm.
Although normofibrinogenemia is a key factor in hemorrhagic failure in cardiac surgery, fibrinogen supplementation does not correct defects that are not related to fibrin generation, such as lack of thrombin or platelet dysfunction.
| Coagulation factors (I) |
|
Fresh frozen plasma (FFP). Indications :
- Intracranial haemorrhage on VKA
- Plasmapheresis
- Massive hemorrhage
- Non-availability of isolated coagulation factors
In other situations, risks (hemodynamic overload, pulmonary complications, allergic reaction) are likely to outweigh benefits
Fibrinogen :
- Indicated in cases of bleeding when its level is < 2 g/L (prophylactic administration ineffective)
- Dosage: 25-50 mg/kg (3 gm needed to increase serum level by 1 g/L)
- Administration: freeze-dried preparation
- FFP: contains only 2 g/L (0.4 - 0.5 g/U)
|
Prothrombin complex concentrates
Lyophilised prothrombin complex concentrates (PCCs, formerly PPSBs) include vitamin K-dependent factors. They can be divided into 3 categories [11,37].
- Concentrates of 3 factors: factors II, IX, X (Prothromplex HT® ); used mainly in North America to antagonise AVKs.
- Concentrates of 4 factors: factors II, VII, IX, X (Prothromplex T® , Kanokad® , Kaskadil® , Cofact® , Beriplex® , Octaplex® ); dosage: 20-25 IU/kg in case of persistent bleeding, 30-50 IU/kg in case of intracranial bleeding on AVK.
- Activated concentrates: factors II, VII, IX, X, some of which are in activated form (FEIBA® , Factor eight inhibitor bypassing activity, Autoplex-T® ); dosage: 50-100 IU/kg.
The concentration of each factor varies greatly between preparations. Some also contain antithrombin III and/or proteins C and S. Concentration factors is 25 times higher than in plasma. The units (IU) correspond to the units of factor IX contained in the preparation (see Table 8.10) [9].
Their indications are primarily prevention or treatment of haemorrhage in haemophiliacs A and B and reversal of AVKs in the event of acute bleeding, particularly intracranial. Factor consumption by massive bleeding requires replacement when loss exceeds 200-250% of blood volume [1]. Four-factor concentrates may be a partial antidote to anti-Xa agents (rivaroxaban, apixaban), but not to anti-thrombin agents (dabigatran) (see Antagonism) [10,14,23]. Activated concentrates (FEIBA® ) only make sense in presence of factor VIII and IX inhibition [12], but may be active as an antagonist to dabigatran [10]. PCCs are only fully active if temperature, blood calcium and acid-base balance are optimal. As the half-life of the factors they contain is longer than that of the newer oral anticoagulants, there is a significant risk of thrombosis; therefore, it is recommended that patients be placed on a low prophylactic dose of heparin. Concomitant administration of vitamin K (Konakion® ) is essential when bleeding is due to a AVK [10]. Compared to FFP, PCCs do not present an infectious risk, do not require blood grouping, and provide the required factors quickly and without haemodynamic overload. However, they do carry a thromboembolic risk, particularly in their activated form (FEIBA® ), where the incidence of vascular thrombosis can be as high as 10% [38]. In cardiac surgery, PCCs are more effective than FFP in reducing rate of transfusion (OR 2.4) and refractory bleeding (OR 1.51) [9]. They reduce blood loss and platelet transfusions more than rFVIIa [13]. However, current data are insufficient to recommend routine use of PCCs instead of FFP, particularly because of the risk of hypercoagulability and vascular thrombosis [30].
Activated Factor VII
Recombinant factor VIIa (rFVIIa, NovoSeven® ) is the fastest and most effective way to generate thrombin if prothrombin levels are maintained. It acts on coagulation by two different mechanisms bypassing factors VIII and IX (see Figure 8.1).
- Complex formation with tissue factor (TF) released from vascular lesions; the TF-rFVIIa complex activates the coagulation cascade by stimulating factor X to Xa and causes massive thrombin production (extrinsic pathway);
- Binding to platelets, which activate the conversion of factor X to Xa.
rFVIIa is indicated in congenital haemophilia A and B, Glanzmann thrombasthenia, and factor VII deficiency. It is possible that rFVIIa may be a partial antagonist of anti-thrombin (dabigatran) or anti-Xa (rivaroxaban) agents, but it has a short half-life and does not restore factors IX and X, or factor II (prothrombin), which are only present in PCC or FFP (see Table 8.10) [10]. The recommended dosage is 90 mcg/kg (at the current price of US$ 1.5 per mcg), repeated after 2 hours. Laboratory monitoring is possible by measuring PT and TC/TFC of the thromboelastogram. Outside of these recognised indications, the use of rFVIIa has rapidly spread to various fields that do not meet the recommended indications (off-label use), but sometimes lead to bleeding that is difficult to control: cardiac surgery, polytrauma, liver transplantation, intracranial haemorrhage under AVK, etc. A plethora of publications have appeared in the literature, reporting the potential success of these new applications. However, no data from controlled trials show a significant gain in mortality in these off-label indications [41]. In any case, the substance can only be effective if four elements are controlled [17].
- Platelet count > 70,000/mcL ;
- Blood calcium > 1 mmol/L ;
- Fibrinogenemia ≥ 2 g/L ;
- Haemoglobin ≥ 80 g/L.
A supraphysiological dose of rFVIIa can induce disseminated intravascular thrombosis [3]. The rate of arterial thrombosis on rFVIIa is about 2%, but a meta-analysis found an incidence of thromboembolic events of up to 20% and an increase in stroke in cardiac surgery, while the efficacy of the substance in reducing mortality is not proven [24,25]. Risk of acute renal failure is greater than with PCCs [13].
In the absence of large randomised controlled trials, the use of rFVIIa outside the haemophilia syndrome remains in the off-label domain of the substance and must be clinically judged on a case-by-case basis according to the balance of risks and benefits. It is a last chance measure in the event of uncontrollable haemorrhage despite use of all haemostatic means, including surgery, endoscopy and interventional radiology, but it is in no way a prophylactic measure, especially as its cost is prohibitive (approximately €1,000 for one dose) [36].
Factor XIII
Factor XIII is responsible for the fibrin polymer network strength and has anti-fibrinolytic properties. In cardiac surgery, the administration of FXIII (Fibrogammin P® 10-30 IU/kg) after protamine reduces postoperative blood loss, but its clinical usefulness has been partially investigated to date [36]. It appears to be effective in patients with lowered factor XIII levels (decreased peak clot firmness on thromboelastogram), which frequently occurs in cases of significant blood loss or large wounds (burns, spinal surgery, liver surgery) [8]. As in the previous case, it is only justified when other treatments have failed to stop bleeding.
An imbalance between thrombin and factor XIII is typical of coagulation activation with a depletion of FXIII stores. It is characterised by presence of fibrin monomers in circulation, which can be measured in laboratory. Thus, detection of fibrin monomers makes it possible to predict a haemostasis defect due to a lack of factor XIII; the PT and aPTT are not modified by the lack of factor XIII. Factor XIII substitution (10-20 IU/kg, 750-1,500 IU for an adult) restores CMF on the thromboelastogram and reduces blood loss [22].
von Willebrand factor
Von Willebrand factor (vWF) has three main effects: on one hand, it interacts with the GP Ib/V/IX receptor of platelets; on the other hand, it binds to collagen in the subendothelium; and keeps Factor VIII in solution. It therefore anchors platelets to the injured vascular wall. It may be lacking due to congenital or acquired deficiency (neoplasia, severe aortic stenosis) (see Chapter 21, Haematological Diseases). Substitution with 1 IU/kg (Haemate® , Wilate® ) increases the level by about 2%. It is recommended that 30 IU/kg be given pre-operatively, 20-30 IU/kg during surgery, and 20-30 IU/kg 3 x/d for 5 days. The product half-life is 14-17 hours. The level required for normal haemostasis is > 80% of the normal level. In the special case of severe aortic stenosis, acquired von Willebrand disease affects more than 25% of patients with a rheumatic type of vavulopathy. However, these patients do not have an increased bleeding risk during valve replacement in ECC. AVR restores functional completeness of the VWF and suppresses spontaneous bleeding symptoms [5].
| Coagulation factors (II) |
|
Prothrombin Complex Concentrates (PCC): contains vitamin K dependent factors (II, VII, IX, X) in varying concentrations depending on the product. Indications :
- Haemophilia A and B
- Intracranial haemorrhage on VKA
- Replacement of factors II, VII, IX, X in massive hemorrhage (if possible objectified)
by specific tests)
- Possible antagonists of anti-Xa agents (?)
Activated factor VII (rFVIIa, NovoSeven® ). Given its cost and risk of thrombosis, rFVIIa is only indicated as a rescue measure in cases of massive bleeding, once the following have been controlled: platelets > 70,000/mcL, blood calcium > 1 mmol/L, fibrinogenemia ≥ 2 g/L, Hb ≥ 80 g/L.
- Formal indication: haemophilia A and B, Glanzman thrombasthenia
- Possible antagonist of anti-thrombin or anti-Xa agents (?)
- Off-label use: rescue in massive bleeding,afterfailure of
other measures
- Dosage: 90 mcg/kg, repeatafter2 hours
Factor XIII: the presence of fibrin monomers during massive bleeding indicates a factor XIII deficiency. Rescue measure if other therapies fail.
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- ASMIS LM. Coagulation factor concentrates. In: MARCUCCI CE, SCHOETKKER P, ed. Perioperative hemostasis. Coagulation for anesthesiologists. Heidelberg: Springer, 2015, 177-204
- BOLLIGER D, GÖRLINGER K, TANAKA KA. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology 2010; 113:1205-19
- BROWN C, JOSHI B, FARADAY N, et al. Emergency cardiac surgery in patients with acute coronary syndromes: a review of the evidence and perioperative implications of medical and mechanical therapeutics. Anesth Analg 2011; 112: 277-99
- CASBARD AC, WILLIAMSON LM, MURPHY MF, et al. The role of prophylactic fresh frozen plasma in decreasing blood loss and correcting coagulopathy in cardiac surgery. A systematic review. Anaesthesia 2004; 59: 550-8
- CASONATO A, SPONGA S, PONTARA E, et al. Von Willebrand factor abnormalities in aortic valve stenosis: pathophysiology and impact on bleeding. Thromb Haemost 2011; 106:58-66
- CHOWDARY P, SAAYMAN AG, PAULUS U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol 2004; 125: 69-73
- FENGER-ERIKSEN C, JENSEN TM, KRISTENSEN BS, et al. Fibrinogen substitution improves whole blood clot firmnessafterdilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomised, placebo-controlled clinical trial. J Thromb Haemost 2009; 7: 795-802
- FERRARIS VA, BROWN JR, DESPOTIS GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011; 91:944-82
- FITZGERALD J, LENIHAN M, CALLUM J, et al. Use of prothrombin complex concentrate for management of coagulopathyaftercardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth 2018; 120:928-34
- GHADIMI K, LEVY JH, WELSBY IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth Analg 2016; 122:1287-300
- GOODNOUGH LT, SHANDER A. Current status of pharmacologic therapies in patient blood management. Anesth Analg 2013; 116: 15-34
- GUYATT GH, AKL EA, CROWTHER M, et al. American College of Chest Physicians antithrombotic therapy and prevention of thrombosis panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: 7S-47S
- HARPER PC, SMITH MM, BRINKMAN NJ, et al. Outcomes following three-factor inactive prothrombin complex concentrate versus recombinant activated factor VII administration during cardiac surgery. J Cardiothorac Vasc Anesth 2018; 32:151-7
- HEIDBUCHEL H, VERHAMME P, ALINGS M, et al. European Heart Rythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015; 17:1467-507
- HIIPPALA ST, MYLLYLA GJ, VAHTERA EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg 1995; 81: 360-5
- JEPPSSON A, WALDÉN K, ROMAN-EMANUEL C, et al. Preoperative supplementation with fibrinogen concentrate in cardiac surgery: a randomized controlled study. Br J Anaesth 2016; 116:208-14
- KARKOUTI K, BEATTIE WC, WIJEYSUNDERA DN, et al. Recombinant factor VIIa for intractable blood lossaftercardiac surgery: A propensity score-matched case-control analysis. Transfusion 2005; 45:26-34
- KARKOUTI K, CALLUM J, CROWTHER MA, et al. The relationship between fibrinogen levelsaftercardiopulmonary bypass and large volume red cell transfusion in cardiac surgery: an observational study. Anesth Analg 2013; 117: 14-22
- KARLSSON M, TERNSTRÖM I, HYLLNER M, et al. Prophylactic fibrinogen infusion reduces bleedingaftercoronary artery ECC. A prospective randomised pilot study. Thromb Haemost 2009; 102: 137-44
- KLEIN AA, ARNOLD P, BINGHAM RM, et al. AAGBI guidelines: the use of blood components and their aletrnatives 2016. Anaesthesia 2016; 71:829-42
- KOR DJ, STUBBS JR, GAJIC O. Perioperative coagulation management - fresh frozen plasma. Best Pract Res Clin Anaesthesiol 2010; 24: 51-64
- KORTE WC, SZADKOWSKI C, GÄHLER A, et al. Factor XIII substitution in surgical cancer patients at high risk for intraoperative bleeding. Anesthesiology 2009; 110: 239-45
- KOZEK-LANGENECKER SA, AHMED AB, AFSHARI A, ALBALADEJO P, et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. First update 2016. Eur J Anaesthesiol 2017; 34: 332-95
- LEE AI, CAMPIGOTTO F, RAWN JD, et al. Clinical significance of coagulation studies in predicting response to activated recombinant facteor VII in cardiac surgery patients. Br J Haematol 2012; 157:397-400
- LEVI M, LEVY JH, ANDERSEN HF, et al. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010; 363: 1791-800
- LEVY JH, SZLAM F, TANAKA K, SNIECIENSKI RM. Fibrinogen and hemostasis: A primary hemostatic target for the management of acquired bleeding. Anesth Analg 2012; 114:261-74
- MURAD MH, STUBBS JR, GANDHI MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion 2010; 50: 1370-83
- RAHE-MEYER N, LEVY JH, MAZER CD, et al. Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double-blind phase III study of haemostatic therapy. Br J Anaesth 2016; 117:41-51
- RAHE-MEYER N, SOLOMON C, HANKE A, et al. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery. Anesthesiology 2013; 118: 40-50
- RALEIGH L, COLE SP. Con: Factor concentrate usage in cardiac surgery - A paucity of data limits their universal adoption. J Cardiothorac Vasc Anesth 2018; 32:1068-71
- RANUCCI M, BARYSHNIKOVA E. Fibrinogen supplementationaftercardiac surgery: insights from Zero-Plamsa trial (ZEPLAST). Br J Anaesth 2016; 116:618-23
- RANUCCI M, BARYSHNIKOVA E, CRAPELLI GB, et al. Randomized, double-blinded,placebo-controlled trial of fibrinogen concentrate supplementationaftercomplex cardiac surgery. J Am Heart Assoc 2015; 4:e002066
- RANUCCI M, PAZZAGLIA A, BIANCHINI C, et al. Body size, gender, and transfusions as determinants of outcomeaftercoronary operations. Ann Thorac Surg 2008; 85: 481-7
- ROBACK JD, CALDWELL S, CARSON J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion 2010; 50: 1227-39
- ROSSAINT R, BOUILLON B, CERNY V, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care 2010; 14:R52
- ROZENTAL T, SHORE-LESSERSON L. Pharmacologic management of coagulopathy in cardiac surgery: An update. J Cardiothorac Vasc Anesth 2012; 26:660-79
- SIÉ P, SAMAMA CM, GODIER A, et al. Surgeries and invasive procedures in patients treated long-term with an oral anti-IIa or anti-Xa direct anticoagulant. Proposals from the Perioperative Haemostasis Interest Group (GIHP) and the Haemostasis and Thrombosis Study Group (GEHT). Ann Fr Anesth Réanim 2011; 30: 645-50
- SMITH MM, ASHIKHMINA E, BRINKMAN NJ, et al. Perioperative use of coagulation factor concentrates in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2017; 31:1810-9
- TANAKA KA, KOR DJ. Emerging haemostatic agents and patient blood management. Best Pract Res Clin Anaesthesiol 2013; 27: 141-60
- WEBER CF, GÖRLINGER K, MEININGER D. Point-of-care testing: a prospective randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012; 117: 531-47
- YANK V, TUOHY CV, LOGAN AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011; 154: 529-40