The different types of prosthetic valves are divided into mechanical and biological valves. There are three main types of mechanical prostheses.
- Ball valve (Starr-Edwards), consisting of a Silastic™ ball oscillating in a cage made up of two arches fixed to a ring (steel) covered with Teflon™; when the valve is open, the flow passes around the ball; when the valve is closed, it is leakproof (Figure 11.34); the effective opening area is small and the size is considerable. This prosthesis is no longer implanted, but a few patients still have it.
Video: Starr valve in aortic position (long-axis view); the ball moves back and forth longitudinally during the cardiac cycle.
Figure 11.34: Starr-Edwards™ valve. Hemodynamically, it has 2 different openings: the annulus (large circular opening) and the annulus fibrosus (annular opening between the annulus fibrosus and the aortic or ventricular wall, with a smaller effective area) [From: Zipes DP et al, eds. Braunwald's Heart Disease, 7th ed. Philadelphia: Elsevier Saunders, 2005. Figure 57.47 ].
- Oscillating disc valve (Medtronic-Hall™, Bjork-Shiley™): a pyrolocarbon disc oscillates about an eccentric pivot in a ring, allowing passage through two unequal-sized orifices; when closed, the valve has small leaks at the edges or at the central pivot (Figure 11.35). These prostheses are no longer used except in certain artificial hearts, but they are occasionally found.
Video: Bjork-Shiley™ valve in mitral position; disc oscillates during cardiac cycle.
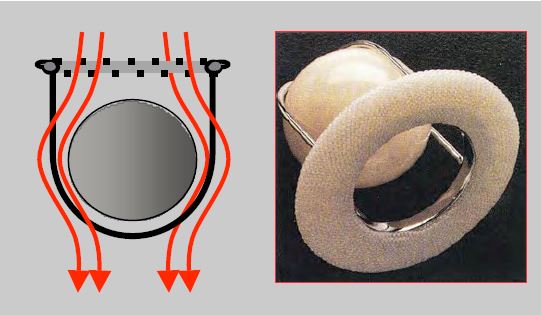
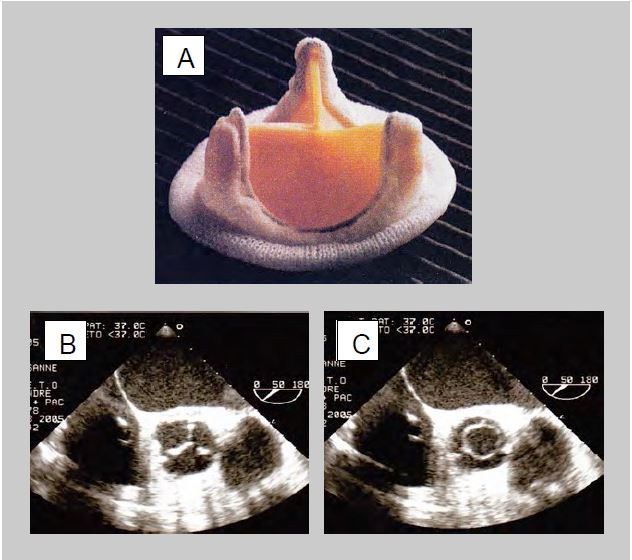
- Double-finned valve (St. Jude Medical™, Carbomedics™, ATS™, On-X™) in titanium and pyrolocarbon: When open, the fins form an angle of 85° with the flow, which passes through 2 crescent-shaped orifices on each side and a small central orifice with a rectangular cross-section where the gradient is greater. In the closed position, the fins form an angle of 25-30° with the plane of the ring and have small leaks at the edges (fin-ring junction) and at the pivots (Figure 11.36). The On-X™ unalloyed pyrolocarbon prosthesis is fixed in the supra-annular position, saving one size, i.e. 2 mm in diameter. Flow and gradient are measured in the two lateral orifices and not in the small central orifice where the gradient is up to 40-50% higher [43,47]. These stents have a lifetime of at least 25 years.
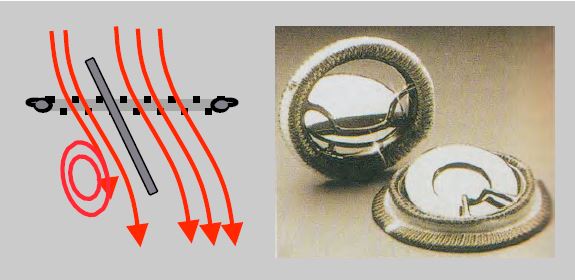
Figure 11.35: Oscillating disc valve (Medtronic-Hall™, Bjork-Shiley™). When opened, the valve has one large and one small opening. The angle of the disc is 70° to the flow axis, creating resistance in the large orifice and vortices in the small orifice. On closure, leaks occur at the edges and, in some models, at the pivot axis in the centre of the valve. disc [From: Zipes DP et al, eds. Braunwald's Heart Disease, 7th ed. Philadelphia: Elsevier Saunders, 2005. Figure 57.47] .
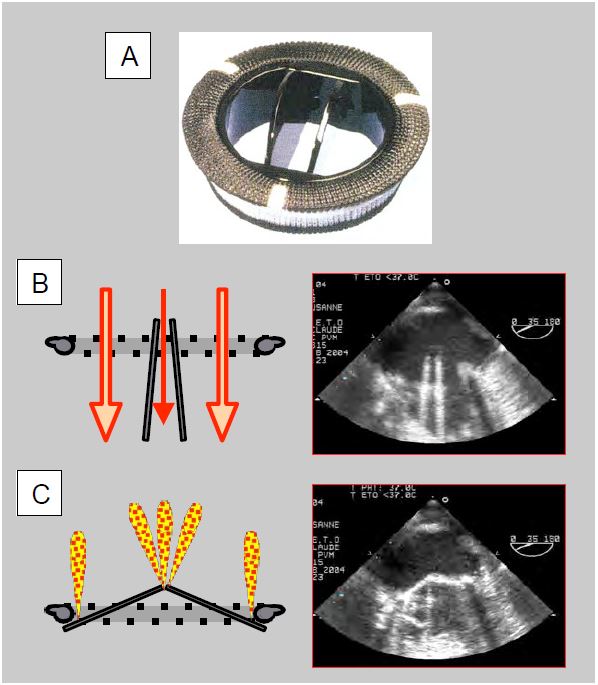
Figure 11.36: Double valve (St Jude Medical™, Carbomedics™, ATS™). A: mechanical double-finned valve (taken from: Zipes DP et al, eds. Braunwald's Heart Disease, 7th ed. Philadelphia: Elsevier Saunders, 2005. Figure 57.47). B: Diagram and TEE image of an open double-fin valve in the mitral position. When open, the wings form an angle of 85° with the flow, which passes through 2 crescent-shaped orifices on each side and a central slit of rectangular cross-section where the gradient is greater. C: Diagram and TEE image of a bi-fin valve closed in the mitral position. In the closed position, the leaflets are at an angle of 25° to the annulus plane and show autolavage leaks at the edges and at the fulcra. The prosthesis is placed with its fins in an anti-anatomical position in relation to the mitral leaflets, therefore the profile in which the two fins are visible on TEE is between 30° and 80°.
The rings are covered with Dacron™ or braided Teflon™ to allow sutures to be anchored. Double-finned valves are the most commonly used as they offer the best haemodynamic profile. Mechanical prostheses typically have autolavage leaks, the purpose of which is to prevent the deposition of fibrin and platelets on the free edge of the ribs and on the inner part of the annulus, as this could block movement or prevent sealing. These leaks represent a fraction of regurgitation ≤ 5%.
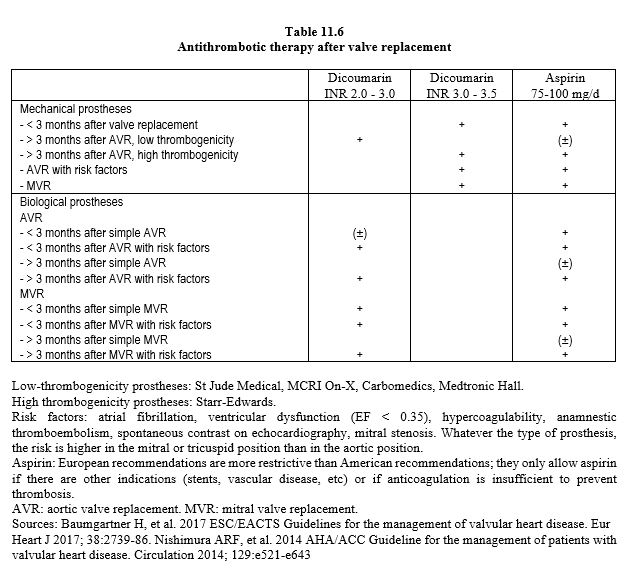
On colour Doppler, they come from the inside of the annulus and the hinge; they are thin and extend over 1-2 cm. These characteristics distinguish them from paravalvular leaks originating outside the annulus, which are larger and originate from a visible solution of continuity between the annulus and the wall. Mechanical prostheses require lifelong anticoagulation, aiming for an INR of 3.0-3.5 for MVRs and 2.0-3.0 for AVRs (possibly 1.5-2.0 for On-X valves in the aortic position), with the addition of aspirin (75-250 mg/d) in the absence of contraindications (Table 11.6) [4,27,31,42]. Because they are less thrombogenic, the new prostheses do not require aspirin [36].
Biological valve prostheses
Biological valves are made from porcine valve tissue or bovine pericardium; this category also includes homografts (human tissue) and heterografts (animal tissue). The tissue is prepared in a glutaraldehyde bath, which reduces its antigenicity but alters its mechanical properties; the valves become less flexible, calcify and degenerate over time. They have a functional life of 12 to 20 years, which is inversely proportional to the patient's age, being longer in the elderly. In subjects aged 60 years, bioprostheses have an average life of 11.4 years in the mitral position and 17.6 years in the aortic position [1]. Bioprostheses may or may not be mounted on a frame, the diameter of which defines the size of the prosthesis, but the actual internal diameter is 2 mm smaller in porcine valves (Biocor™, Hancock™ II) and 1 mm smaller in pericardial valves (Perimount™, MagnaEase™); it is identical only in valves with leaflets implanted outside the frame (Mitroflow™, Trifecta™) [3]. Bioprostheses require anticoagulation for only 3 months (INR 2.5), after which endothelialisation is complete [4,27]. Omitting this period of anticoagulation triples the risk of thromboembolic events [40]. Long-term use of aspirin is controversial unless there are other reasons (coronary artery disease, stents, arterial disease, etc.) [4,42], but is most often recommended as routine [29] (Table 11.6) (Figure 11.37).
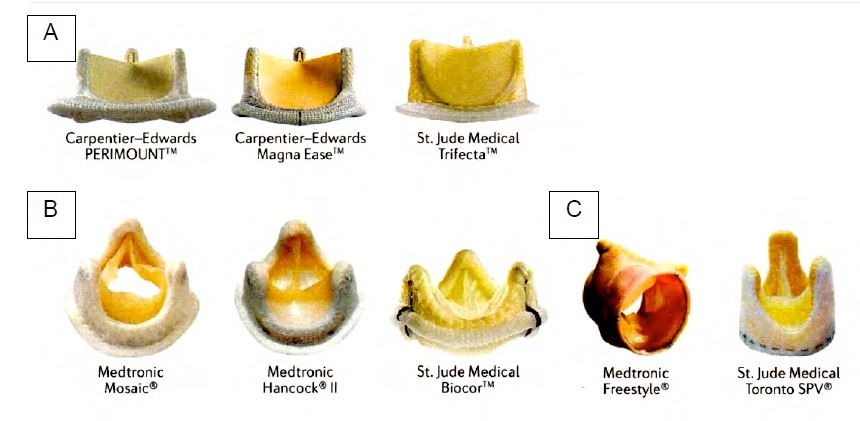
Figure 11.37: Bioprosthetic heart valves. A: Valves assembled with bovine pericardial leaflets. B: Valves assembled with porcine pericardial leaflets. C: stentless valves [taken from: Arsalan M, Walther T. Durability of prostheses for transcatheter aortic valve implantation. Nat Rev Cardiol 2016; 13:360-7].
- Conventionally mounted bioprostheses; porcine aortic valve (Biocor™, Hancock™ II) or bovine pericardial valves (Carpentier-Edwards Perimount™ or MagnaEase™, Trifecta™, Mitroflow™) mounted on a metal ring and suspended by their commissures on 3 vertical pins. The valves have a certain opening inertia due to their relative rigidity. For the same size, the effective orifices of these valves are smaller than those of mechanical valves and unmounted bioprostheses; their gradients are also higher. When closed, these valves are airtight or have small leaks, usually centrally but sometimes at the commissures. For the same size, bovine pericardial valves have a slightly better haemodynamic performance than porcine valves and a slightly lower attrition rate; their mean survival without revision is 19 years (Figure 11.38) [1,12].
Video: Biological prosthesis in aortic position (short-axis view); the 3 cusps and 3 suspension posts are clearly visible.
Video: Biological prosthesis in mitral position; only 2 of the 3 struts and 3 leaflets are visible in an echocardiographic cross-section; the most anterior strut encroaches into the LV outflow tract.
Figure 11.38: Biological valve prosthesis (from: Zipes DP et al, eds. Braunwald's Heart Disease, 7th ed. Philadelphia: Elsevier Saunders, 2005. Figure 57.49). A: Carpentier-Edwards bioprosthesis. B: Prosthesis closed in the aortic position; the 3 leaflets are in the same position as in the native valve. C: Open prosthesis in the aortic position; the opening is practically circular and easily measured by planimetry (TEE views short aortic axis 50°).
- New generation of mounted bioprostheses; a thinner ring (Carpentier-Edwards Perimount Magna™, in bovine pericardium) increases the internal diameter by 2 mm for the same external diameter; its special configuration allows supra-annular fixation, saving one size compared to a standard prosthesis. Reduced spike size (St. Jude Biocor™, porcine valve) and special treatment of the porcine valve (Medtronic Mosaic™) provide a mechanical advantage, reduce the risk of calcification and increase longevity (70% at 20 years) [13]. Out-of-frame leaflets (Mitroflow™, Trifecta™) increase the effective opening area [3].
- Stentless bioprostheses (Freestyle™, Toronto SPV™, CryoLife™, Freedom Solo™, based on a porcine valve with an outer cover of polyester fabric): for the same diameter, they have a larger opening and a lower gradient than other valves. Their configuration and the absence of an annulus allow them to be used only in the aortic position; the diameter of the patient's aortic annulus and that of the sino-tubular junction must not differ by more than 10% for the prosthesis to be correctly suspended at the commissures (Figure 11.39). These valves are technically more difficult to implant because the fixation points on the prosthesis and on the patient's aortic annulus must be strictly equidistant, but their rate of endocarditis and thrombosis is very low. They are generally indicated for patients over 60 years of age with small aortic rings.
Video: Short-axis view of a stentless bioprosthesis in aortic position; presence of the 3 cusps but absence of posts.
Video: Long-axis view of a stentless bioprosthesis in aortic position with colour flow; there is no regurgitation in diastole or flow restriction in systole. Normal postoperative presence of a sleeve haematoma around the aortic root.
- Bioprostheses without fixation (sutureless) (Perceval S™, 3F Enable™, Intuity Elite™): Their implantation requires ECC, aortotomy and excision of the native valve, but is faster because the valve is placed by self-expansion or balloon inflation as with percutaneous prostheses (TAVI). The prosthesis is fixed under direct visual control, which reduces the risk of paravalvular leakage compared with TAVI (15% vs 45%) [11]. The results are equivalent to a conventional bioprosthesis, but the duration of ECC is halved. This technique is well suited for small aortic rings (< 2.2 cm), high-risk cases (age > 80 years) and fast-track patients [17,33].
- Homografts: mitral valve or aortic valve (with ascending aortic sleeve) harvested from human cadavers and preserved in liquid nitrogen (-196°C). Although they have an accelerated wear rate, they are resistant to infection and are particularly indicated for valve replacement in cases of endocarditis [23]. Their problem is their limited availability and the risk of malposition during implantation, which is more difficult than that of an attached valve.
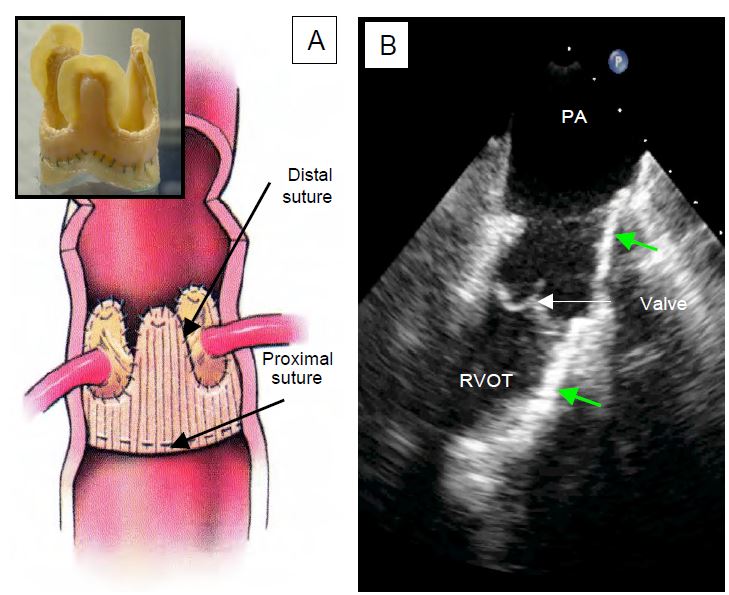
- Heterografts: The bovine jugular vein is a valved conduit that can be prepared with glutaraldehyde and cryopreserved (Contegra™) to replace the right outflow tract (RVOT, pulmonary valve and PA trunk) [44].
Video: Contegra™ heterograft in the pulmonary position (long-axis view of the valve and pulmonary artery in a short-axis image of the aortic arch).
- Autograft: The Ross procedure consists of replacing the aortic valve with the patient's own pulmonary valve, which is replaced by a heterograft or homograft (see Aortic valve surgery and Figure 11.146). The main advantage of this technique is that it allows for growth, as the aortic neo-valve develops at the same pace as the child does.
- Valved LV conduit - descending aorta: In the case of multiple reoperations, it is possible to short-circuit the aortic root by means of a Dacron tube fitted with a prosthetic valve which is implanted in the apex of the LV and anastomosed termino-laterally to the descending aorta. The operation is performed via a left thoracotomy without ECC [9,28].
The major problem with bioprostheses is their long-term wear rate. With current prostheses, structural degeneration of the valve (tears, thickening, fibrosis, calcification) occurs in 4-8% of cases after 5 years, 10-15% after 10 years, 21% after 15 years and 50-60% after 20 years; reoperation for valve replacement is required in 2-4% of cases after 10 years and 15-20% after 20 years [1,8,12]. In the aortic position, deterioration is moderate when the mean gradient is 20-40 mmHg (VLVOT /VAo ratio 0.25-0.35) and severe when it is > 40 mmHg (VLVOT /VAo < 0.25); regurgitation is moderate or severe, respectively [8]. The durability of bioprostheses is poorer in young people whose cardiac erethism wears the valve prematurely and in patients with impaired calcium metabolism (renal failure, dialysis, parathyreosis) [1].
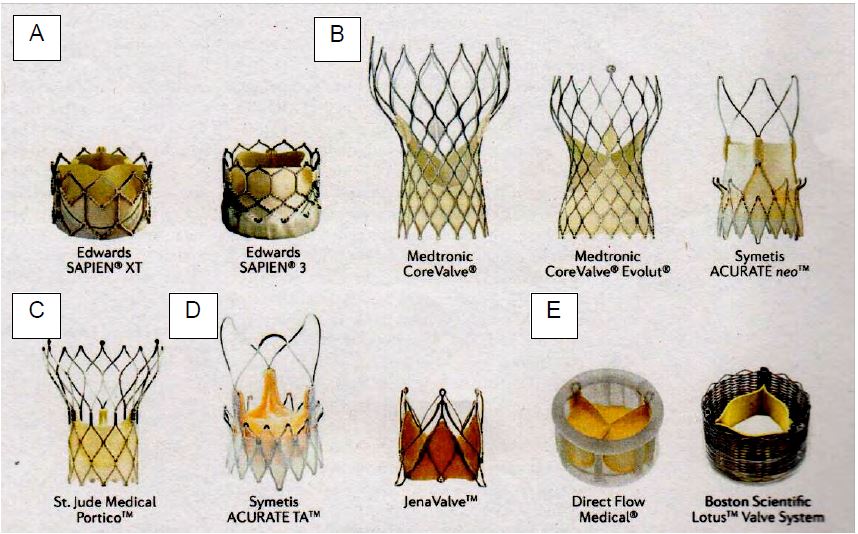
Endovascularly implanted bioprostheses (TAVI, transcatheter aortic valve implantation) (Figure 11.40) are indicated in patients with an intermediate or high surgical risk, preferably elderly patients, whose life expectancy does not exceed 5-10 years (see TAVI). Long-term outcomes are therefore not critical. The rate of deterioration with TAVI is 2-10% at 5 years; in the short term, the new prostheses give results that are almost comparable to those of surgically implanted valves [1]. However, laboratory fatigue tests show that the durability of TAVI valves is about half that of surgical valves (7.8 versus 16 years) [26]. In addition, the risk of valve thrombosis is around 7% with TAVI compared to less than 1% with surgical replacement [21]. If necessary, reoperation can be performed endovascularly (valve-in-valve technique) (see Chapter 10 Other valve procedures without ECC).
Figure 11.40: There are many types of valve available for transcatheter implantation (TAVI). A: Balloon-expandable prostheses. B: Self-expanding porcine pericardial prosthesis. C: Self-expanding bovine pericardial stent. D: Self-expanding prosthesis in native porcine valve. E: alternative bovine pericardial prosthesis [taken from: Arsalan M, Walther T. Durability of prostheses for transcatheter aortic valve implantation. Nat Rev Cardiol 2016; 13:360-7].
Haemodynamics of prostheses
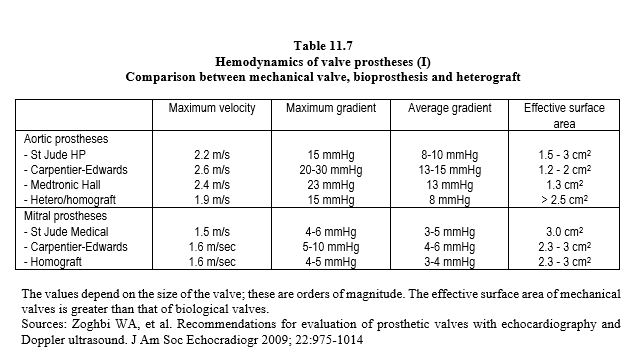
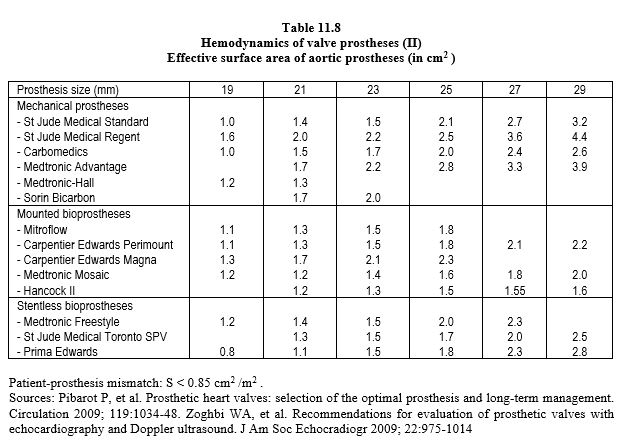
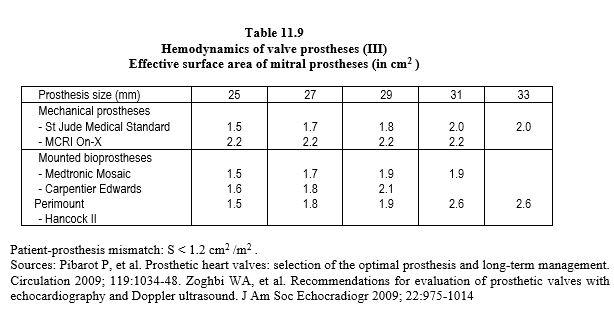
Each type of prosthesis has its own haemodynamic characteristics. However, the opening surface area of a prosthetic valve is smaller than the normal surface area of the native valve, varying from 1.1 cm2 (aortic bioprosthesis 19) to 3.5 cm2 (mechanical mitral valve 33). As a result, the pressure gradient is significant; the mean gradient varies from 3 mmHg (St Jude mitral valve) to 15 mmHg (aortic bioprosthesis) (Tables 11.7, 11.8 and 11.9) [6,29,31,42]. For the same size, the gradients are in ascending order: homografts < stentless bioprostheses < mechanical valves < stented bioprostheses. Stents fixed in the supra-annular position have a lower gradient than those fixed in the native annulus. The maximum gradient is considered abnormal if it is > 35 mmHg at the aortic level and > 10 mmHg at the mitral level. Most of the technical data for each type of prosthesis can be found in the reference Echo prothèses 2009.pdf (see under Guidelines) and on the open access website: www.valveguide.ch [15].
- The geometry of stents induces a pressure recovery phenomenon: the law of conservation of energy means that pressure decreases as velocity increases in the constricted zone, but the kinetic energy is converted back into pressure as soon as the flow slows beyond the constriction [38]. Doppler calculates ΔP from Vmax, which corresponds to the narrowest point of the flow (vena contracta); this zone is generally very short (1-3 mm) and pressure rises rapidly in the downstream chamber (see Figure 11.44). This phenomenon is more pronounced in prostheses than in native valves, and increases with valve size because the turbulence created by small prostheses causes a loss of kinetic energy. For these reasons, the mean gradient is a better criterion than the maximum gradient [47].
- The greater the mismatch between the prosthesis and the receiving chamber, the lower the pressure recovery because energy is wasted in turbulence; thus, a large aorta reduces the downstream pressure because the energy loss due to turbulence is greater. Therefore, the inflated gradient measured by Doppler tends to underestimate the true orifice area in small prostheses and large aortas [47].
- In the aortic position, the velocity in the LV outflow tract is often greater than 1.5 m/s after valve replacement in cases of stenosis and LVH. It is therefore essential to use the modified Bernoulli equation ΔP = 4 · (V22 - V12 ) and not its simplified version (ΔP = 4 · V2 ). Failure to subtract Vmax in the LVOT leads to an overestimation of the gradient by 9 to 30 mmHg.
- Surface calculations such as the half-pressure time or the equation of continuity (see Chapter 25, Half-pressure time, Equation of continuity) assume that the orifices are circular, which they are not in mechanical prostheses. Therefore, the half-pressure time is not applicable to assess the surface area of a mitral prosthesis. In the continuity equation used to measure aortic valve area, the reference value is generally the stroke volume in the LVOT (SAo = (SLVOT · ITVLVOT ) / ITVVAo ). However, the cross-sectional area of the LVOT is oval rather than circular, and the long-axis slice used in echocardiography to assess its diameter actually measures the smallest diameter, which is 17% smaller, underestimating the area by an average of 24% [5,16]. In addition, measuring the diameter of the LVOT is difficult because of the brightness and shadows of the prosthesis.
- The gradient is increased by 40% in the central part of double-finned valves, so it must be measured with the Doppler axis in one of the two wide lateral orifices; in biological prostheses it is measured in the centre of the valve.
- On leaving bypass, the flow is accelerated due to catecholaminergic stimulation and the transient increase in systolic volume (transfusion, high flow, extrasystole); the artificially accelerated velocity through the prosthesis tends to underestimate the real surface area.
- Low pressure in the aortic root (vasoplegia, intra-aortic balloon pump, ascending aortic aneurysm) increases the gradient of an aortic prosthesis by reducing the downstream pressure. In case of IABP, this difference is of the order of 30-40 mmHg.
- Raise the ΔP at different points on the prosthesis; this is particularly important in double valve prostheses as the velocity through the central slot is higher than through the 2 large lateral holes.
- Measure Vmax in the LVOT in the aortic position and calculate the ratio VLVOT /VAo; if it is > 0.4, the valve is functioning normally.
- Calculate the effective surface area of the prosthesis in relation to the body surface area; if it is < 0.85 cm /m2 in the aortic position or < 1.2 cm /m2 in the mitral position, the size of the valve is insufficient for the patient's cardiac output (see Complications below, Patient-prosthesis mismatch).
- Assess the extent of any valvular regurgitation that increases systolic or diastolic volume.
- Compare echocardiographic measurements with haemodynamic measurements: is there aortic arterial hypotension (IABP), increased stroke volume (Swan-Ganz, PiCCO), excess catecholamines β (cardiac output)?
- As a last resort, measure the peak-to-peak gradient with a needle between the LV and the ascending aorta in AVR or between the LV and the LA in MVR.
Operative mortality
The average mortality of valve replacement remains high in all categories of patients combined [22,34,39]:
- AVR 1.5 - 3.1%
- MVR 2.5 - 5.5%
- PVR < 1.0 - 1.5%.
- TVR 7-9%.
- AVR + aortic root 8%
In elective, low-risk cases, mortality is 1.5% for AVR, 3-4% for MVR and < 1% for mitral valve surgery (Society of Thoracic Surgeons 2017). Patients undergoing valve replacement between the ages of 55 and 65 have a 15-year mortality rate that varies by valve type [18,32,40].
- Biological AVR 36% (6%/patient/year)
- Mechanical AVR 32% (4%/patient/year)
- Biological MVR 50%
- Mechanical MVR 45%.
The independent variables associated with increased mortality are listed in descending order of importance (OR: odds ratio) [34]:
- Emergency surgery OR 2.11
- Age > 70 years OR 1.88
- Reoperation OR 1.61
- Endocarditis OR 1.59
- Associated coronary artery disease OR 1.58
In general, valve repair has a 50% lower mortality rate than valve replacement. The prosthesis itself is responsible for only 40-50% of long-term mortality; bleeding is the cause of death in 25% of cases with mechanical valves and 12% with bioprostheses [20].
Selection criteria and the future
The goal is obviously to replace the native valve with the largest prosthesis possible to achieve the lowest possible transvalvular gradient. The effective orifice area (EOA) is the cross-sectional area of the prosthesis available for flow. In the presence of stenosis or regurgitation, it is measured against that of the vena contracta. If the native annulus is too narrow, a stentless prosthesis or a supra-annular implantable prosthesis can be chosen to increase the size. If this is not possible, the aortic annulus can be decalcified and the aortic root enlarged with a Dacron™ patch. This manoeuvre is difficult to perform in the mitral position due to the risk of annulus rupture, circumflex artery injury and posterobasal wall tearing of the LV with catastrophic consequences [31].
Mechanical valves have a long lifespan (>20 years without injury) but require permanent anticoagulation (INR 2.0-3.5), whereas bioprostheses do not require anticoagulation once endothelialisation is complete (>3 months). On the other hand, biological valves are subject to structural degradation, which reduces their lifetime. About 30% of 1st generation conventional bioprostheses fail after 10 years [2].
Video: Degeneration of a bioprosthesis in the mitral position; presence of pannus obstructing the orifice and immobility of the leaflet.
Video: Degeneration of a bioprosthesis in the mitral position; colour flow shows small regurgitation and tight stenosis in the diastolic passage.
The rate rises to 50% after 15 years, requiring reoperation with a high mortality rate (4-15%) [35]. The new 3rd generation bioprostheses, such as Perimount™ or Trifecta™, have a significantly lower degeneration rate than the older models (10-15% at 10 years, 21% at 15 years) [12,18,32]. On the other hand, the rates of embolism, thrombosis, endocarditis and long-term mortality are virtually identical for mechanical valves and bioprostheses [40]. The choice is therefore between two alternatives.
- Mechanical prostheses: risks associated with anticoagulation, but very long prosthesis survival;
- Bioprosthesis: valve survival limited to about fifteen years, but no anticoagulation.
Bioprostheses are indicated in patients with a high risk of bleeding, in patients over 65 years of age, in patients who are unable to adhere to long-term treatment, and in young women who wish to become pregnant, on the understanding that reoperation will be necessary after about 15 years. The criteria for mechanical prostheses are a life expectancy of more than 20 years (age < 65), no contraindication to anticoagulation, anticoagulation already prescribed for other reasons (e.g. atrial fibrillation) and a high risk of accelerated prosthesis degeneration (hypercalcemia, renal failure, young age) [31,42].
In the case of bioprostheses, the main choice is between mounted and stentless prostheses. Stentless prostheses are generally reserved for older patients with small aortic rings and suitable anatomy [32]. The rate of deterioration of homografts is identical to that of bioprostheses, but their resistance to infection makes them a good solution in the event of endocarditis. In children, the Ross procedure (autografting of the aortic valve using the patient's own pulmonary valve and replacement of the latter with a homo/heterograft, see Figure 11.146) allows the aortic valve to grow, but its operative mortality is higher than that of simple AVR (3-4%) and the reoperation rate after the age of 12 is 10% for the aortic valve and 20-30% for the pulmonary homograft [10,41].
At 10 years, the overall incidence of mechanical prosthesis-related problems is 3% and anticoagulation-related problems are 7% [19]; however, 15-30% of bioprostheses are no longer functional due to structural deterioration [2,32]. After 20 years, a comparison of patients with mechanical and biological valves shows no difference in mortality, but survival without a major event or valve replacement is much higher in patients with mechanical valves [30].
Prostheses are most commonly implanted in the aortic or mitral position, less commonly in the tricuspid position. MVRs are preferably performed with mechanical valves because bioprostheses in the mitral position have three particular disadvantages.
- Mounted bioprostheses tend to partially obstruct the outflow tract with one of their three pins;
- Stentless prostheses cannot be used because there is no anatomical tubular structure to fit them;
- The attrition rate of bioprostheses in the mitral position is higher than that of the same prostheses in the aortic position because of the greater haemodynamic stress.
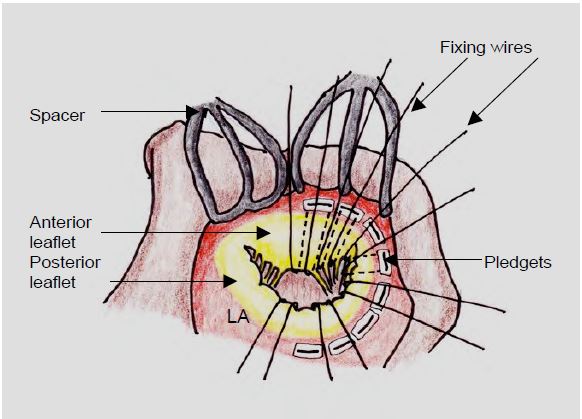
During MVR, mechanical prostheses with double winglets are oriented in an anti-anatomical position (right-left and not anterior-posterior), and those with tilting discs are oriented with the largest opening on the posterior side, so that the flow is oriented in its physiological axis towards the posterolateral wall and not towards the apex [31]. Visualisation of the movement of the two winglets on TEE is generally optimal in the mid-oesophageal position at 30-80°. The mitral subvalvular apparatus forms the internal skeleton of the LV. If it is completely resected to implant the prosthesis, the LV will gradually dilate and become spherical, and its systolic function will be significantly reduced. This is why the leaflets, chords and pillars are preserved as much as possible when the prosthesis is fixed (Figure 11.41) [45]. This is easy as long as they are not too shrunken, fibrotic or restrictive. In this case, at least the 3rd cords should be preserved.
Figure 11.41: Preservation of the leaflets and subvalvular apparatus during mitral valve replacement (MVR). The prosthesis fixation wires are passed under the leaflets and chords (or what remains of them after resection) and implanted in the mitral annulus on pledgets, small Teflon rectangles that hold the wire to the atrial side of the annulus. This arrangement can sometimes give the prosthesis some apparent mobility on ultrasound.
In the tricuspid position, where the pressure regime is low, mechanical prostheses have less inertia than bioprostheses, but have a 1%/year risk of thrombosis [24]. The long-term survival of bioprostheses is better than in the mitral or aortic position because of the lower mechanical stress. However, pericardial stents should be avoided because of the stiffness of their leaflets. Regardless of the type, the valve frame does not match the curvature of the LV wrapped around it and hinders the longitudinal peristaltic movement of the LV. After tricuspid valve replacement, the rate of right heart failure is 28% (9% after valvuloplasty) and mortality is 11% [37].
| Heart valve prostheses |
|
Mechanical prostheses have a long lifespan (> 25 years) but require permanent anticoagulation (INR 2.0-3.0 after AVR and 3.0-3.5 after MVR). Current models are double finned (St. Jude Medical, Carbomedics, ATS). Biological valves only require anticoagulation for 3 months, but have a limited lifetime due to structural degradation; their attrition rate is 15-30% at 10 years and 20-50% at 15 years (depending on the model).
Preferred indications for mechanical prostheses are
- Life expectancy > 20 years, young and active patient
- No contraindication to anticoagulation or anticoagulation already prescribed
- Risk of accelerated degeneration of the bioprosthesis (hypercalcemia, renal failure)
Preferred indications for bioprostheses
- Age > 65 years
- Contraindication to anticoagulation (high risk of bleeding, poor compliance, treatment, pregnancy)
The effective surface area of prostheses is smaller than that of native valves. As a result, the pressure gradient is higher. The gradient varies from model to model, in ascending order: homograft < stentless bioprosthesis < mechanical valve < stented bioprosthesis. The mean gradient is considered abnormal if it is > 20 mmHg at the aortic level and > 5 mmHg at the mitral level. The gradient is often overestimated at the end of bypass surgery for several reasons:
- High stroke volume, high cardiac output, sympathetic stimulation (transfusions), infusions, catecholamines);
- Low aortic pressure (vasoplegia, IABP);
- Echocardiographic calculation of Vmax influenced by the geometry of the prosthesis;
- After AVR: ignorance of LVOT Vmax: ΔP = 4 ·(VVAo 2 - VLVOT 2)
-After AVR, the ratio VLVOT /VVao must be > 0.4; a lower value indicates stenosis.
In elective cases with no particular risk, the operative mortality rate for AVR is 2%, for MVR 3-5% and for mitral valve surgery ≤ 1%.
|
References
- ARSALAN M, WALTHER T. Durability of prostheses for transcatheter aortic valve implantation. Nat Rev Cardiol 2016; 13:360-7
- BANBURY MK, COSGROVE DM, WHITE JA, et al. Age and valve size effect on the long-term durability of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg 2001; 72:753-9
- BAPAT VN, ATTIA R, THOMAS M. Effect of valve design on the stent internal diameter of a bioprosthetic valve. JACC Interv 2014; 7:115-27
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BAX JJ, DELGADO V. Advanced imaging in valvular heart disease. Nat Rev Cardiol 2017; 14:209-23
- BETTEX D, CHASSOT PG. Transoesophageal echocardiography in anaesthesia and intensive care. Paris: Masson, Williams & Wilkins, 1997, 454 pp.
- BURSTOW DJ, NISHIMURA RA, BAILEY KR, et al. Continuous wave Doppler echocardiographic measurement of prosthetic valve gradients. A simultaneous Doppler-catheter correlative study. Circulation 1989; 80:504-11
- CAPODANNO D, PETRONIO AS, PRENDERGAST B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the EAPCI endorsed by the ESC and the EACTS. Eur Heart J 2017; 38:3382-90
- CRESTANELLO JA, ZEHR KJ, DALY RC, et al. Is there a role for the left ventricle apical - aortic conduit for acquired aortic stenosis? J Heart Valve Dis 2004; 13:57-62
- DAVID TE. The Ross procedure at the crossroad. Circulation 2009; 119:207-9
- D'ONOFRIO A, MESSINA A, LORUSSO R, et al. Sutureless aortic valve replacement as an alternative treatment for patients belonging to the "gray zone" between transcatheter aortic valve implantation and conventional surgery: a propensity-matched, multicenter analysis. J Thorac Cardiovasc Surg 2012; 144:1010-8
- DVI D, BOURGUIGNON T. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aorticvalves. Circulation 2018; 137:388-99
- EICHINGER WB, HETTICH IM, RUZICKA DJ, et al. Twenty-year experience with the St.Jude medical Biocor bioprosthesis in the aortic position. Ann Thorac Surg 2008; 86:1204-10
- FERNANDES V, OLMOS L. NAGUEH SF, et al. Peak early diastolic velocity rather than pressure half-time is the best index of mechanical prosthetic mitral valve function. Am J Cardiol 2002; 89:704-9
- FRANK M, GANZONI G, STARCK C, et al. Lack of accessible data on prosthetic heart valves. Int J Cardiovasc Imaging 2016; 32:439-47
- GASPAR T, ADAWI S, SACHNER R, et al. Three-dimensional imaging of the left ventricular outflow tract: impact on aortic valve area estimation by the continuity equation. J Am Soc Echocardiogr 2012; 25:749-57
- GERSAK B, FISCHLEIN T, FOLLIGUET TA, et al. Sutureless, rapid deployment valves and stented bioprosthesis in aortic valve replacement: recommendations of an International Expert Consensus Panel. Eur J Cardio-Thorac Surg 2016; 49:709-18
- GOLDSTONE AB, CHIU P, BAIOCCHI M, et al. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N Engl J Med 2017; 377:1847-57
- HAMMERMEISTER KE, SEHTI GK, HENDERSON WG, et al. A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or a bioprosthesis. N Engl J Med 1993; 328:1289-95.
- HAMMERMEISTER KE, SEHTI GK, HENDERSON WG, et al. Outcomes 15 years after valve replacement with a mechanical versus bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol 2000; 36:1152-8
- HOLMES DR, MACK MJ. Aortic valve bioprostheses. Leaflet immobility and valve thrombosis. Circulation 2017; 135:1749-56
- IUNG B, BARON G, BUTCHART EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J 2003; 24:1231-43
- KARCHMER AW LONGWORTH DL. Infections of intracardiac devices. Infect Dis Clin North Am 2002; 16:477-89
- LANCELLOTTI P, MOURA L, AGRICOLA E, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010; 11:307-32
- MAHMOOD F, MATYAL R, MAHMOOD F, et al. Intraoperative echocardiographic assessment of prosthetic valve: a practical approach. J Cardiothorac Vasc Anesth 2018; 32:823-37
- MARTIN C, SUN W. Comparison of transcatheter aortic valve and surgical bioprosthetic valve durability: a fatigue simulation study. J Biomech 2015; 48:3026-34
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- ODONKOR P, STANSBURY LG, GAMMIE JS, et al. Anesthetic management of patients undergoing aortic valve bypass (api-aortic conduit) surgery. J Cardiothorac Vasc Anesth 2012; 26:148-60
- OH JK, SEWARD JB, TAJIK AJ. The echo manual. 3rd edition. Philadelphia, Lippincott Williams & Wilkins, 2006, 189-242
- OXENHAM H, BLOOMFIELD P, WHEATLEY DJ, et al. Twenty-year comparison of a Björk-Shiley mechanical heart valve with porcine bioprostheses. Heart 2003; 89:715-22
- PIBAROT P, DUMESNIL JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation 2009; 119:1034-48
- RAHIMTOOLA SH. Choice of prosthetic heart valve in adults. An update. J Am Coll Cardiol 2010; 55:2413-26
- RAMAKRISHNA H, PATEL PA, GUTSCHE JT, et al. Surgical valve replacement - Clinical update on recent advances in the contemporary era. J Cardiothorac Vasc Anesth 2016; 30:1733-41
- RANKIN JS, HAMMIL BG, FRERGUSON B, et al. Determinants of operative mortality in valvular heart surgery. J Thorac Cardiovasc Surg 2006; 131:547-57
- RUEL M, KULIK A, RUBENS FD, et al. Late incidence and determinants of reoperation in patients with prosthetic heart valves. Eur J Cardiothorac Surg 2004; 25:364-70
- SINGH M, SPORN ZA, SCHAFF HV, PELLIKKA PA. ACC/AHA versus ESC Guidelines on prosthetic heart valve management. J Am Coll Cardiol 2019; 73:1707-18
- SINGH SK, TANG GH, MAGANTI MD, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg 2006; 82:1735-41
- STEWART SF, NAST EP, ARABIA FA, et al. Errors in pressure gradient measurement by continuous wave Doppler ultrasound: type, size and age effects in bioprosthetic aortic valves. J Am Coll Cardiol 1991; 18:769-79
- STS- Society of Thoracic Surgeons National Cardiac Surgery Database, 2017. https://www.sts.org/site/defaut/files/documents/ ACSD_ExecutiveSummary2017Harvest4_RevisedReport.pdf
- SURI RM, SCHAFF HV. Is tissue valve the preferred option for patients aged 60 years and older? Circulation 2013; 128:1372-80
- TAKKENBERG JJM, KLIEVERIK LMA, SCHOOF PH, et al. The Ross procedure: a systematic review and meta-analysis. Circulation 2009; 119:222-8
- VAHANIAN A, ALFIERI O, ANDREOTTI F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012; 33:2451-96
- VANDERVOORT PM, GREENBERG NL, POWELL KA, et al. Pressure recovery in bileaflet heart valve prostheses. Localized high velocities and gradients in central and side orifices with implications for Doppler-catheter relation in aortic and mitral position. Circulation 1995; 92:3464-72
- WALTHER T, LEHMANN S, FALK V, et al. Prospective randomized evaluation of stented xenograft hemodynamic function in the aortic position. Circulation 2004; 110:II74-8
- YUN KL, SINTEK CF, MILLER DC, et al. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: effects on left ventricular volume and function. J Thorac Cardiovasc Surg 2002; 123:707-14
- ZABALGOITIA M. Echocardiographic assessment of prosthetic heart valves. Curr Probl Cardiol 2000; 25:157-66
- ZOGHBI WA, CHAMBERS JB, DUMESNIL JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocradiogr 2009; 22:975-1014