Mitral stenosis accounts for approximately 10% of adult valvular disease (prevalence 0.1-0.2%). It is mainly caused by rheumatic fever and calcific degeneration. While ARF remains the leading cause in developing countries, calcific degeneration is becoming a major aetiology in developed countries and its incidence increases with age [4,5]. Other causes are rare: endocarditis, congenital disease (parachute valve), toxic substances (methysergide), systemic diseases, atrial myxoma, abnormal serotonin metabolism [2]. In ARF and calcific degeneration, the structures undergo such changes that pure stenosis is rare (25-40% of cases) and is usually associated with some degree of insufficiency. Although the mitral valve is most commonly affected, ARF can also affect the aortic valve.
Anatomically, mitral stenosis in ARF is characterised by commissural fusion and thickening of the distal part of the two leaflets; the central part of the anterior leaflet generally retains some flexibility. Secondary calcification is common in elderly patients. Typically, the subvalvular apparatus is also affected by the disease: the chords are thickened, fused and shortened. Occasionally, fibrosis and calcification invade the basal part of the ventricular myocardium, affecting segmental kinetics. Women are three times more likely to be affected than men [1,3]. Calcific degeneration of the mitral valve is a progression of mitral annular calcification (MAC) in the leaflets. This condition is related to the generalised vascular calcification seen in advanced atheromatosis, aortic stenosis and senescent renal failure [4].
The cardiac silhouette of pure mitral stenosis is typical; it alone summarises the entire pathology (Figure 11.86).
- The left atrium is very dilated: its wall is thin, the atrial appendage is distended; the interatrial septum bulges into the RA; the very slow flow increases the risk of thrombus.
- The left ventricle is small: mostly normal in configuration and function, myocardial mass is reduced and end-diastolic volume is low.
- The mitral valve is thickened, retracted, not very mobile and more or less calcified; the subvalvular apparatus is restrictive and some chords are fused.
- The size of the right cavities depends on the degree of pulmonary hypertension: hypertrophy and dilatation of the RV, tricuspid insufficiency and dilatation of the RA; the interventricular septum bulges into the LV in diastole and represents an additional obstacle to filling it.
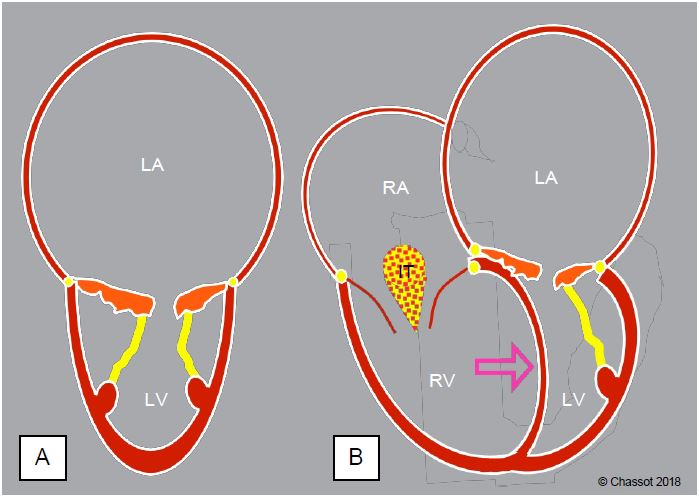
Figure 11.86: Silhouette of the ventricles in mitral stenosis. A: The LV is small and the LA is dilated, the leaflets and subvalvular apparatus are thickened and retracted. B: Upstream congestion has induced postcapillary pulmonary hypertension responsible for LV dilatation; secondary tricuspid insufficiency (TI) is almost always present. The interventricular septum bulges into the LV (arrow), further reducing diastolic filling. This septal displacement is less pronounced in the absence of pulmonary hypertension because the LV fills more rapidly than the RV. In associated mitral or aortic regurgitation, the LV remains closer to its normal size and may even dilate to some extent.
Echocardiography shows this peculiar silhouette of a small LV and a large RV. Commissural fusion immobilises the free edges of the valve and narrows its orifice (Figure 11.87); this deformation takes the form of a cone protruding into the LV (Figure 11.88). As the stiffness of the valve prevents it from closing normally, insufficiency is common. In the presence of a significant MI, the LV retains a more normal size, which is a functional advantage.
Video: Typical 4-cavity view of mitral stenosis in ARF; the mitral leaflets are fused at the commissures and open little, but their central part remains flexible; the LV is small while the LA is gigantic.
Video: Commissural fusion in mitral stenosis.
Video: Three-dimensional view from the LV of severe mitral stenosis; the valve takes the shape of a cone plunging into the ventricle; its opening in diastole is only a slit.
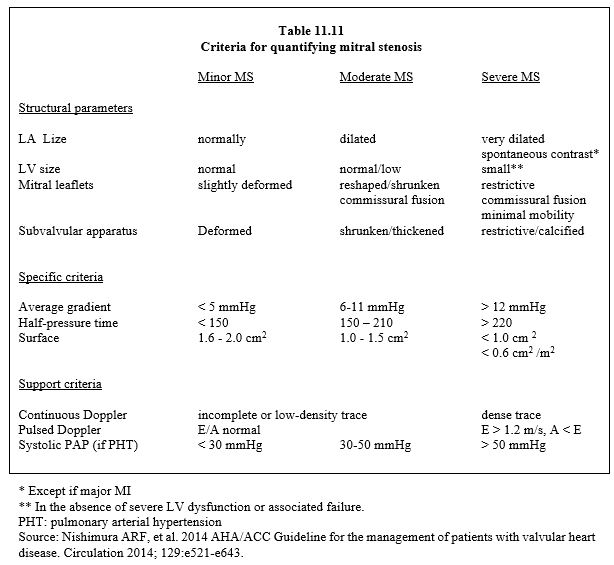
The normal mitral surface area is 4 to 6 cm2 and its gradient is minimal (≤ 4 mmHg). Stenosis is classified into three degrees (Table 11.11):
- Mild stenosis: area 1.6 - 2.0 cm2, the patient is asymptomatic.
- Moderate stenosis: area 1.0 - 1.5 cm2, mean gradient 5-11 mmHg, PAPsyst 30-50 mmHg, symptoms only on exertion.
- Severe stenosis: area < 1.0 cm2 (< 0.6 cm2 /m2), mean gradient ≥ 12 mmHg, PAPsyst > 50 mmHg at rest; the PLA required to maintain cardiac output at rest is ≥ 20 mmHg; symptoms are usually present with the slightest exertion or at rest.
A mitral valve area of less than 0.4 cm2 is not compatible with survival.
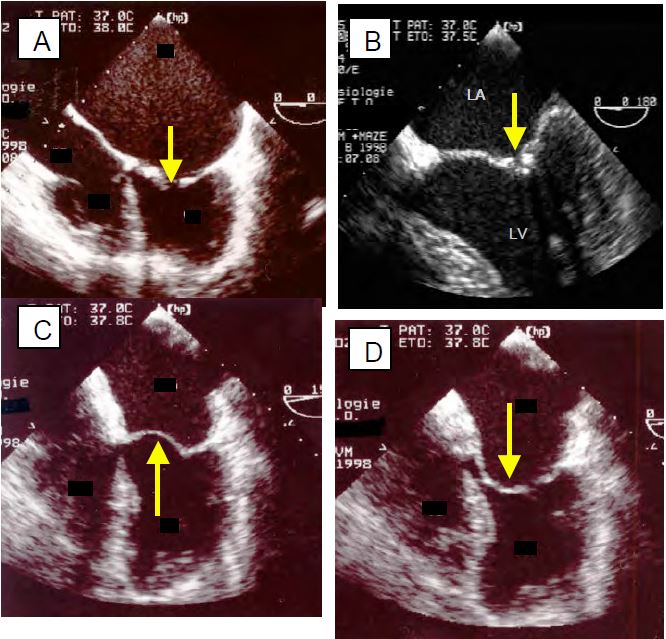
Figure 11.87: Mitral stenosis (MS) in ARF (TEE images). A: 4-cavity view; the LA is huge, the LV is small; commissural fusion holds the leaflets in diastole and the opening (arrow) is minimal. B: very tight commissural fusion of the two leaflets C: the body of the anterior leaflet, which remained flexible in the ARF, bulges into the LA during systole. D: The body of the anterior leaflet bulges into the LV during diastole (hockey stick deformity).
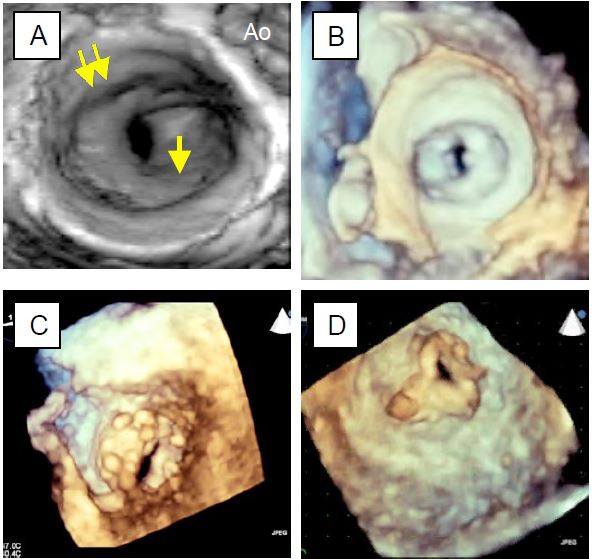
Figure 11.88: 3D echocardiographic images of mitral stenosis. A: View from the LA; the orifice is at the bottom of a cone due to fusion of the commissures (single arrow), below the plane of the annulus (double arrow). B: Atrial view of mitral stenosis; the orifice is a slit limited by the commissural fusions. C: View from the LV; the valve is deformed into a cone, the orifice is only a slit. D: View from the left side of a secondary stenosis of a mitral bioprosthesis, showing the triangular shape of the bioprosthesis.
| Mitral stenosis |
| Definition of degrees of mitral stenosis:
- Mild surface stenosis 1.6 - 2.0 cm2 ΔPmean < 5 mmHg
- Moderate regurgitation 1.0 - 1.5 cm2 ΔPmean 6-11 mmHg
- Narrow area stenosis < 1 cm2 (0.6 cm2 /m2) ΔPmean > 12 mmHg
Etiology: Essentially ARF or calcifying degeneration characterised by:
- Commissural fusion, distal leaflet thickening, secondary calcifications
- Fusion and narrowing of the subvalvular apparatus
- Large dilatation of the LA, small or normal size of the LV
|
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- BONOW RO, BRAUNWALD E. Valvular heart disease. In: ZIPES DP, et al, eds. Braunwald's heart disease. A textbook of cardiovascular medicine. 7th edition. Philadelphia: Elsevier Saunders, 2005, 1553-632
- BRAUNWALD E. Valvular heart disease. In: BRAUNWALD E. ed. Heart disease. Philadelphia, WB Saunders Co, 1997, 1007-76
- NKOMO VT, Gardin JM, SKELTON TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005-11
- SUD K, AGARWAL S, PARASHAR A, et al. Degenerative mitral stenosis. Unmet need for percutaneous interventions. Circulation 2016; 133:1594-604
- TM, EL-GHONEIMI, ARMSTRONG S, et al. Mitral valve repair and replacement for rheumatic disease. J Thorac Cardiovasc Surg 2000; 119:53-60