Total anomalous pulmonary venous return (total APVR)
Due to a misconnection with the LA during embryonic development, all the pulmonary veins drain directly or indirectly into the RA (L-to-R shunting) in four main ways (Figure 14.33) [3].
Due to a misconnection with the LA during embryonic development, all the pulmonary veins drain directly or indirectly into the RA (L-to-R shunting) in four main ways (Figure 14.33) [3].
- Supracardiac type: the posterior collector (PC), which drains the venous blood from both lungs into a system located behind the atria, is connected to the superior vena cava system (innominate vein, azygos vein or SVC) by a vertical vein (45% of cases).
- Cardiac type: the PC drains into the RA via the coronary sinus (25% of cases).
- Infracardiac type: the PC drains by the transdiaphragmatic route into the portal system via an often stenotic descending vein (25% of cases)
- Mixed type: the pulmonary veins anastomose directly with the RA on the right side and with the innominate vein on the left side (5%).
These venous return systems are in most cases insufficient, stenotic or compressed by their surroundings. In cases of totally blocked APVR, an obligatory ASD allows the venous and arterialised blood to mix, and supplies the LA and systemic circulation (R-to-L shunt), which therefore receives mixed blood. Clinical strategy is dominated by the extent of venous return obstruction resulting from this unusual anatomy. Diagnosis is difficult at birth as the clinical picture (respiratory distress, acidosis and multiple organ failure) is indicative as well of pulmonary disease or infection. If the obstruction is severe, neonates present with refractory cyanosis (SpO2 < 75%) and pulmonary venous hypertension (post-capillary PHT) from their first days of life. Pulmonary oedema is common and NO is contraindicated. The RA and RV are highly dilated, while the LA and LV are small. Total pulmonary blood flow is very high (Qp:Qs 2-3:1) even if PVR remains relatively low. Systemic flow is largely dependent on the ductus arteriosus whose flow is provided by the already overloaded RV [6].
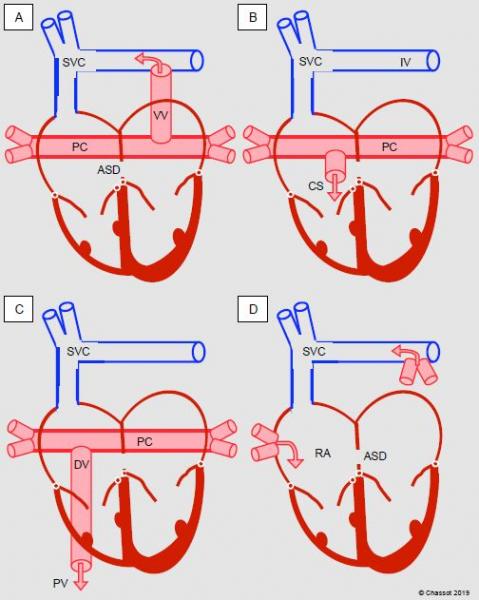
Figure 14.33: Diagram showing total anomalous pulmonary venous return. A: supracardiac type, into the innominate vein (IV) via a vertical vein (VV). B: cardiac type, via the coronary sinus (CS). C: infracardiac type, transdiaphragmatic into the portal vein (PV) via a descending vein (DV). D: mixed type. In the first three cases, a posterior collector (PC) drains venous blood from both lungs into a system located behind the atria.
A blocked total APVR is the only genuine emergency in neonatal cardiac surgery. Surgical correction is required in the first hours of life with CPB and deep hypothermia, the pulmonary collector is anastomosed directly into the LA, the anomalous connection is occluded, and the ASD is closed. If the ASD is restrictive, it can be widened preoperatively by Rashkind balloon septostomy enabling the infant’s immediate survival. If the venous return is not obstructed, surgical correction may be postponed up to 6-8 weeks. The surgical mortality rate is 8-13% [2]. In the preoperative phase, it may be necessary to stabilise neonates’ haemodynamics and ventilation: inotropes (dobutamine, milrinone-epinephrin), diuretic, intubation, mechanical ventilation, prostaglandins, etc. If the pulmonary blood flow is too high, ventilation is used to increase PVR by hypercapnia.
Anaesthesia is achieved by high doses of opiates (fentanyl 50-100 mcg/kg, sufentanil 2.5-10 mcg/kg) combined with midazolam. If neonates’ hearts are anomalous and defective, they are unable to tolerate halogenated agents and often require inotropic support even in the preoperative phase. Transesophageal echocardiography should be used with great caution – it is sometimes even impossible as the probe compresses the pulmonary venous return, which crosses the LA posteriorly. An epicardial echocardiogram may be performed in such instances [6].
Weaning from CPB is associated with three severe phenomena [2].

Figure 14.33: Diagram showing total anomalous pulmonary venous return. A: supracardiac type, into the innominate vein (IV) via a vertical vein (VV). B: cardiac type, via the coronary sinus (CS). C: infracardiac type, transdiaphragmatic into the portal vein (PV) via a descending vein (DV). D: mixed type. In the first three cases, a posterior collector (PC) drains venous blood from both lungs into a system located behind the atria.
A blocked total APVR is the only genuine emergency in neonatal cardiac surgery. Surgical correction is required in the first hours of life with CPB and deep hypothermia, the pulmonary collector is anastomosed directly into the LA, the anomalous connection is occluded, and the ASD is closed. If the ASD is restrictive, it can be widened preoperatively by Rashkind balloon septostomy enabling the infant’s immediate survival. If the venous return is not obstructed, surgical correction may be postponed up to 6-8 weeks. The surgical mortality rate is 8-13% [2]. In the preoperative phase, it may be necessary to stabilise neonates’ haemodynamics and ventilation: inotropes (dobutamine, milrinone-epinephrin), diuretic, intubation, mechanical ventilation, prostaglandins, etc. If the pulmonary blood flow is too high, ventilation is used to increase PVR by hypercapnia.
Anaesthesia is achieved by high doses of opiates (fentanyl 50-100 mcg/kg, sufentanil 2.5-10 mcg/kg) combined with midazolam. If neonates’ hearts are anomalous and defective, they are unable to tolerate halogenated agents and often require inotropic support even in the preoperative phase. Transesophageal echocardiography should be used with great caution – it is sometimes even impossible as the probe compresses the pulmonary venous return, which crosses the LA posteriorly. An epicardial echocardiogram may be performed in such instances [6].
Weaning from CPB is associated with three severe phenomena [2].
- Pulmonary hypertensive crisis (50% of cases) – requires NO•, hyperventilation, FiO2 1.0, magnesium, PGE1. PHT is linked to PVR hyperreactivity, congestive LV failure and/or residual stenosis on the pulmonary venous return. It results in RV failure. High frequency oscillatory ventilation is often highly beneficial [4].
- Overloading of the hitherto hypofunctional and hypoplastic LV – inotropic agents (dobutamine, epinephrin) and an inodilator (milrinone), ECMO in cases of refractory dysfunction. In many cases, sternal closure must be delayed.
- Supraventricular rhythm disorders in 5-20% of cases.
Episodes of arterial desaturation are linked to several factors: pulmonary hypertensive crisis, acute pulmonary edema, low cardiac output, persistent ASD, drainage of the coronary sinus into the LA. The situation may also be complicated by a residual pulmonary venous obstruction (collector stenosis), which adversely affects haemodynamics. A velocity > 1.8 m/s and monophasic flow in the collector on a post-CPB echocardiogram indicate an obstruction. Therapeutic management is dependent on the clinical picture and the severity of the PHT. Although ECMO is often required in cases of emergency surgery, the survival rate of these infants exceeds 90% [5].
Partial anomalous pulmonary venous return (partial APVR)
One or more pulmonary veins, generally on the right side, may take an anomalous path to the RA instead of anastomosing normally with the LA. This usually concerns a right superior pulmonary vein which connects with the root of the SVC into the RA (Figure 14.34). This anomaly is often associated with a sinus venosus-type ASD (see Figure 15.11C).
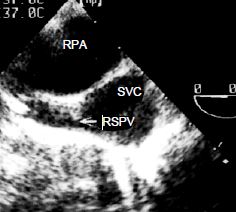
Figure 14.34: Partial anomalous pulmonary venous return. The right superior pulmonary vein (RSPV) flows into the superior vena cava (SVC) just above its anastomosis into the RA. RPA: right pulmonary artery (short-axis view of the ascending aorta at 0° with clockwise rotation of the probe).
The right inferior pulmonary vein may drain into the inferior vena cava (scimitar syndrome), and the left pulmonary veins may drain into the innominate vein or coronary sinus. PAPVR leads to right-sided overload with dilation of the RA and RV. The atrial septum is rarely intact. Surgery is indicated if Qp/Qs is > 2 and if the RV is dilated or in the event of compression of the pulmonary veins and recurrent pulmonary infections. Treatment involves reimplanting the anomalous pulmonary vein in the LA. Surgical correction of anomalous pulmonary venous return combined with a sinus venosus-type ASD involves creating a patch inside the anastomosis of the SVC into the RA so that the arterialised blood is diverted to the LA through the ASD (Warden procedure) [1]. The anaesthesia procedure is the same as for closing an ASD. Postoperatively, the flow should exhibit the systolic and diastolic biphasic morphology of the central venous flows on an echocardiogram. The maximum velocity should remain below 1.0-1.5 m/s. A continuous non-oscillating flow with Vmax > 1.5 m/s indicates prohibitive restriction and requires surgical revision.
Scimitar syndrome
Anomalous right pulmonary venous return into the inferior vena cava is associated with hypoplasia of the right lung whose lower segments receive arterial blood from collaterals of the thoracoabdominal aorta (pulmonary sequestration). This syndrome is named after the resemblance between the chest X-ray and the well-known broad-bladed, curved eastern sabre.
Left superior vena cava
It is not uncommon to find a persistent left superior vena cava (LSVC) on perioperative TEE examination. This occurs in 0.3-5% of the population and in 10% of children with congenital heart disease [7]. It generally drains into the coronary sinus, which is significantly widened (Video and Figure 14.35). The right superior vena cava (RSVC) may be present, hypoplastic or absent.
Video: Enlarged coronary sinus due to a left superior vena cava (see below Figure 14.35).
Prior to CPB, the LSVC is either ligated if it is small or cannulated if its size is large, as it causes a constant flow into the RA and prevents retrograde cardioplegia from reaching the myocardium.
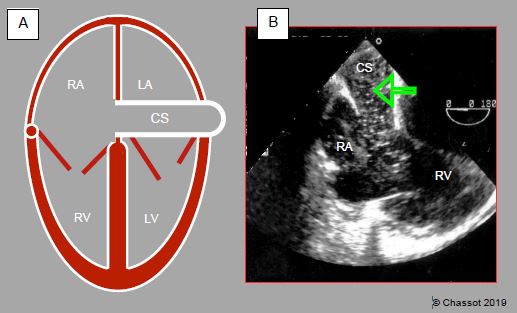
Figure 14.35: Left superior vena cava (LSVC). A: dilation of the coronary sinus (CS) (> 1.5 cm). B: transesophageal echocardiographic view with injection of microbubbles into a vein in the left upper limb – the bubbles appear in the coronary sinus before reaching the RA (arrow).
Although a left SVC has no haemodynamic impact, it entails risks in the event of surgery.
Partial anomalous pulmonary venous return (partial APVR)
One or more pulmonary veins, generally on the right side, may take an anomalous path to the RA instead of anastomosing normally with the LA. This usually concerns a right superior pulmonary vein which connects with the root of the SVC into the RA (Figure 14.34). This anomaly is often associated with a sinus venosus-type ASD (see Figure 15.11C).

Figure 14.34: Partial anomalous pulmonary venous return. The right superior pulmonary vein (RSPV) flows into the superior vena cava (SVC) just above its anastomosis into the RA. RPA: right pulmonary artery (short-axis view of the ascending aorta at 0° with clockwise rotation of the probe).
The right inferior pulmonary vein may drain into the inferior vena cava (scimitar syndrome), and the left pulmonary veins may drain into the innominate vein or coronary sinus. PAPVR leads to right-sided overload with dilation of the RA and RV. The atrial septum is rarely intact. Surgery is indicated if Qp/Qs is > 2 and if the RV is dilated or in the event of compression of the pulmonary veins and recurrent pulmonary infections. Treatment involves reimplanting the anomalous pulmonary vein in the LA. Surgical correction of anomalous pulmonary venous return combined with a sinus venosus-type ASD involves creating a patch inside the anastomosis of the SVC into the RA so that the arterialised blood is diverted to the LA through the ASD (Warden procedure) [1]. The anaesthesia procedure is the same as for closing an ASD. Postoperatively, the flow should exhibit the systolic and diastolic biphasic morphology of the central venous flows on an echocardiogram. The maximum velocity should remain below 1.0-1.5 m/s. A continuous non-oscillating flow with Vmax > 1.5 m/s indicates prohibitive restriction and requires surgical revision.
Scimitar syndrome
Anomalous right pulmonary venous return into the inferior vena cava is associated with hypoplasia of the right lung whose lower segments receive arterial blood from collaterals of the thoracoabdominal aorta (pulmonary sequestration). This syndrome is named after the resemblance between the chest X-ray and the well-known broad-bladed, curved eastern sabre.
Left superior vena cava
It is not uncommon to find a persistent left superior vena cava (LSVC) on perioperative TEE examination. This occurs in 0.3-5% of the population and in 10% of children with congenital heart disease [7]. It generally drains into the coronary sinus, which is significantly widened (Video and Figure 14.35). The right superior vena cava (RSVC) may be present, hypoplastic or absent.
Video: Enlarged coronary sinus due to a left superior vena cava (see below Figure 14.35).
Prior to CPB, the LSVC is either ligated if it is small or cannulated if its size is large, as it causes a constant flow into the RA and prevents retrograde cardioplegia from reaching the myocardium.

Figure 14.35: Left superior vena cava (LSVC). A: dilation of the coronary sinus (CS) (> 1.5 cm). B: transesophageal echocardiographic view with injection of microbubbles into a vein in the left upper limb – the bubbles appear in the coronary sinus before reaching the RA (arrow).
Although a left SVC has no haemodynamic impact, it entails risks in the event of surgery.
- Thromboembolic risk linked to a central venous catheter, which may prompt thrombosis of the coronary sinus – any catheter in the jugular or left subclavian vein must be removed as quickly as possible. If the right SVC is hypoplastic, a right-sided catheter may be virtually occlusive and must also be removed as quickly as possible.
- Problem in terms of venous cannulation during CPB – the LSVC must be drained or excluded if the RSVC is of an appropriate size.
- In the event of retrograde cardioplegia, the infusate leaks into the SVC and does not perfuse the heart.
- Frequently associated AV blocks.
- In the event of drainage into the LA: coronary sinus-type ASD.
- The right-sided route is favoured for implantation of a pacemaker.
| Anomalous venous return |
|
Total anomalous pulmonary venous return (APVR): massive L-to-R shunt due to pulmonary venous return into the RA with several anatomical configurations. Emergency operation in neonates in case of obstruction of the pulmonary collector. Management (already during the preoperative phase):
- Maximise DO2 with mechanical ventilation and high FiO2 - Lower PVR through hyperventilation, alkalosis (no pulmonary vasodilator if stenosis of the collector) - Inotropic support (dobutamine, epinephrin-milrinone) - Potentially ECMO Complications after correcting total APVR on CPB: - PHT - RV failure (overload) - Congestive failure of the hypoplastic LV - Supraventricular arrhythmias Partial APVR: right pulmonary vein drained at the junction of the SVC and RA, often associated with a sinus venosus-type ASD. Left superior vena cava – drains into the widened coronary sinus |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update May 2018
References
- AGGARWAL N, GADHINGLAJKAR S, SREEDHAR R, et al. Warden repair for superior sinus venosus atrial septal defect and anomalous pulmonary venous drainage in children: anesthesia and transesophageal echocardiography perspectives. Ann Card Anaesth 2016; 19:293-9
- BENT ST. Anesthesia for left-to-right shunt lesions. In : ANDROPOULOS DA, et al, eds. Anesthesia for congenital heart disease. Oxford: Blackwell-Futura, 2005, 297-327
- BETTEX D, CHASSOT PG. Transesophageal echocardiography in congenital heart disease. In: BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1186-1212
- BOJAN M, GIOANNI S, MAURAT P, POUARD P. High-frequency oscillatory ventilation and short-term outcome in neonates and infants undergoing cardiac surgery : a propensity score analysis. Crit Care 2011 ; 15 :R259
- HONJO O, Van ARSDELL GS. Cardiovascular procedures : surgical considerations. In : BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1589-608
- NASR VG, DINARDO JA. The pediatric cardiac anesthesia handbook. Oxford: Wiley-Blackwell, 2017; 107-11
- STÜMPER O. Normal and anomaélous venous connections to the heart. In : STÜMPER O, SUTHERLAND GR. Transesophageal echocardiography in congenital heart disease. London, Edward Arnold, 1994, 34-45
