Indirect thrombin inhibitors include non-fractionated heparin (UFH), low molecular weight heparins (LMWH), and polysaccharides such as fondaparinux and idraparinux. These agents are poorly absorbed orally and should be administered parenterally (see Tables 8.1, 8.2, 8.3, 8.11 and 8.12).
Non-fractionated heparin (UFH)
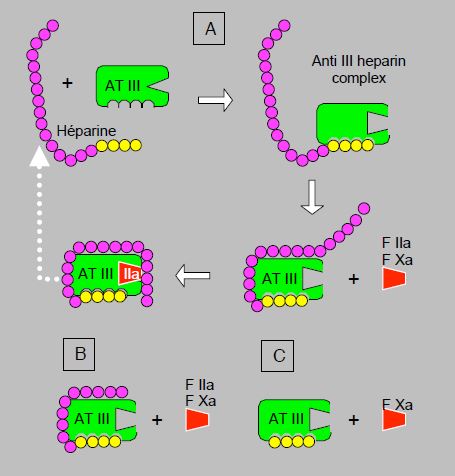
Heparin, originally extracted from liver and now prepared from pig intestinal tissue, is a heterogeneous mixture of polysaccharides (glycosaminoglycan) (molecular weight 8,000-30,000 Da) that bind to antithrombin III (AT III) and accelerate the latter's ability to inhibit factors IIa (thrombin) and Xa, secondarily IXa, XIa and XIIa [1]. It is therefore an indirect agent whose action depends on the presence of sufficient AT III. Without heparin, thrombin and Xa are inhibited by AT III with a half-life of about 1 minute. In the presence of heparin, this reaction is potentiated 700 to 4,000 times [3]. AT III also has indirect anti-inflammatory properties by acting on the production of prostaglandins. The high consumption of AT III during complete heparinisation may therefore activate white blood cells and contribute to the inflammatory cascade after ECC [12]. The action of heparin on AT III is twofold [16]. Firstly, its high-affinity fragment induces a change in the configuration of AT III that significantly increases its affinity for factors IIa and Xa. Secondly, the low-affinity fragment brings factor IIa closer to the site of action of AT III. For AT III to deactivate thrombin (IIa), both mechanisms, conformational change and approximation, are equally important. For deactivation of factor Xa, on the other hand, approximation plays no role (Figure 8.10). These two different mechanisms explain the more or less selective actions of the different heparin derivatives. Non-fractionated heparin (UFH) has an anti-Xa / anti-IIa activity ratio of 1:1. Low molecular weight heparins (LMWH), with a shorter low affinity fragment, have an anti-Xa/anti-IIa ratio of 2-4:1 and fondaparinux pentasaccharide, which contains only the high affinity segment, selectively inhibits factor Xa. Once AT III has bound to the factors, a conformational change decreases its affinity for heparin, which is released and can bind to another AT III molecule. Unfortunately, heparins have no action on thrombin already bound to fibrin within a thrombus.
Figure 8.10: Mechanism of action of indirect thrombin inhibitors (antithrombin III inhibitors, or AT III). A: unfractionated heparin. After binding to its high-affinity fragment for AT III (yellow fragment), heparin induces a conformational change at the AT III site of action, which significantly increases its affinity for factors IIa (thrombin) and Xa. The low-affinity fragment of the heparin chain (purple) serves to bring factor IIa closer to the action site of AT III; this mechanism is not required for factor Xa binding. Once AT III has bound to factors IIa and Xa, a conformational change decreases the affinity for heparin, which is released and can bind to another AT III molecule (white dotted arrow). B: low molecular weight heparin. The low affinity fragment is shorter, which does not allow binding to factor IIa. Factor Xa inhibition is more marked than factor IIa inhibition. C: fundaparinux. The molecule has only the high affinity fragment and can therefore only induce factor Xa binding by AT III.
Intravenous UFH has an immediate onset of action, whereas LMWH derivatives have a 20-60 minute onset of action. The recommended intravenous dosages are higher for thromboembolism than for acute coronary syndrome (ACS) [4].
- Initial bolus: 80 IU/kg, or 5,000 IU (ACS: 60-70 IU/kg);
- Maintenance: 18 IU/kg/h, or 32,000 IU/24 h (ACS: 12-15 IU/kg/h).
The anticoagulant effect of UFH is highly variable due to strong binding to other proteins, macrophages, platelets and endothelium [8]. LMWH and fondaparinux, on the other hand, are much less bound and show a more predictable action. Because of the inter-individual variation of its effect, UFH therefore requires constant monitoring. Its anticoagulant activity correlates well with aPTT and ACT (activated clotting time) (see Monitoring). The shorter derivatives have no predictable effect on these two parameters and are therefore more difficult to monitor, but monitoring is not necessary because their pharmacokinetic profile is more stable.
UFH is resorbed and metabolised by the endothelium and the reticuloendothelial system. The metabolites are inactive and are eliminated renally. The biological half-life is dose dependent: 30 minutes at 25 U/kg, 1 hour at 100 U/kg and 2.5 hours at 400 U/kg [3]. Hypothermia significantly slows down the clearance of heparin. UFH is the most widely used thrombin inhibitor in ECC because of its ease of administration, reversibility with protamine, and the possibility of measuring its activity with coagulation tests such as ACT. Although it has a near linear relationship with serum heparin levels, ACT is not specific for heparin and may show some variability [14]. However, a major limitation of UFH is its inability to inhibit fibrin-bound thrombin in a thrombus. Indeed, thrombin in microthrombi formed on artificial surfaces is beyond the reach of heparin. Direct thrombin inhibitors (lepirudin, bivalirudin, argatroban) have been developed to better inhibit thrombin embedded in the thrombus, but their routine clinical application is limited by their lack of antagonism (see Direct thrombin inhibitors). LMWH and fondaparinux, which also cannot be antagonised, do not have sufficient anti-IIa activity to be useful in extracorporeal circulatory systems.
High concentrations of heparin (≥ 300 IU/kg) induce the release of lipoprotein lipase and hepatic lipase, which hydrolyse plasma triglycerides into free fatty acids (FFA), the level of which rises by 15-20%. These free fatty acids bind to the plasma proteins and displace the substances bound to them, thus increasing the free concentration of these substances and thus their pharmacological activity. This process takes place within minutes [13].
Some patients may be resistant to heparin: despite an adequate dose, aPTT and ACT are not prolonged as expected. There are several reasons for this inadequate response (see Intraoperative Coagulopathy) [4].
- AT III deficiency, particularly common in patients who have been on therapeutic doses of heparin for several days;
- High degree of UFH binding to serum proteins;
- Accelerated clearance of UFH ;
- Excessive factor VIII ;
- Excessive fibrinogen levels.
Low molecular weight heparins (LMWH)
Developed in the 1980s, LMWH are derived from heparin by depolymerisation. Their molecular weight ranges from 2,000 to 9,000 Da. With their high affinity segment, they can induce the necessary configuration change for AT III to bind factors IIa and Xa, but their chain is too short to confront IIa (thrombin) well, which explains their weaker action against it. On the other hand, they have superior anti-Xa activity [1]. Their smaller size explains their low plasma and endothelial binding, which makes their action more predictable. Their effect is poorly assessed by ACT, but can be measured by the anti-Xa assay. Their half-life varies from 4 to 7 hours, but is prolonged to 17 hours when creatinine clearance is < 30 mL/min. Their elimination by the kidneys makes them inappropriate in renal failure and means that doses should be spaced or halved when creatinine clearance is < 50 mL/min [11,15]. LMWHs are less immunogenic than UFH and less likely to induce thrombocytopenia (see HIT). Their dosage varies according to the indication (see Table 8.3). One mg of enoxaparin corresponds to approximately 100 IU of anti-Xa effect [4].
- Prophylaxis (subcutaneous):
- Enoxaparin (Clexane® , Lovenox® )40 mg/d to 30 mg 2 x/d;
- Nadroparin (Fraxiparine® ) 5700 U/d;
- Dalteparin (Fragmin® ) 5'000 U/d.
- Therapeutic (subcutaneous):
- Enoxaparin 1 mg/kg/12h;
- Nadroparin 95 U/kg/12h;
- Dalteparin 120 U/kg/12h.
These dosages are reduced by half in cases of renal failure. Preoperatively, the time between the last subcutaneous injection and surgery is 12 hours for prophylaxis and 24 hours for therapeutic dosing. The first postoperative injection takes place 6-12 hours after the end of surgery in case of prophylaxis, provided that haemostasis is controlled. It is extended to 24-48 hours in case of therapeutic dosage or postoperative bleeding.
Protamine
Protamine, originally extracted from salmon sperm, is now produced by recombinant technology. It is a positively charged polycationic molecule that forms stable complexes with heparin, which is negatively charged. Protamine dissociates heparin-antithrombin complexes. Its plasma half-life is 7 minutes, whereas that of heparin is 60-120 minutes [4]. One mg of protamine (100 IU) neutralises 1 mg of heparin (100 IU). Usually, a dose of protamine is given that is 80% of the heparin dose; an ACT done 5 minutes after the end of protamine administration should be within ±10% of the pre-ECC baseline value. Unfortunately, the relationship between heparin level and post-ECC ACT is non-linear and poorly performing. Dosing based on the patient's actual heparin level (Hepcon/HMS™ system), on thromboelastography (HEPTEM) or on a pharmacokinetic algorithm leads to lower amounts (protamine/heparin ratio 0.5-0.8:1) and thereby reduces the hypocagulability, fibrinolysis and platelet dysfunction generated by protamine (see below) [2]. Neutralisation of the effect of LMWH is possible with 1 mg of protamine per 100 IU of anti-Xa effect (maximum dose: 50 mg), provided that it is less than 8 hours after administration of LMWH; this antagonism is only partial (60%) and the smaller the LMWH molecule, the weaker the antagonism [4]. Protamine does not bind to pentasaccharides (fondaparinux) and therefore cannot antagonise them.
Protamine has several immediate side effects, the incidence of which varies from 1 to 13% of cases (mean 2.6%) [2,6,7].
- Histamine release, which is characterised by significant vasodilation with a decrease in preload and afterload; it is directly proportional to the speed of administration (type I reaction), which is why it is recommended to infuse protamine diluted in 100 mL 0.9% NaCl over 10 minutes. It is very often necessary to accelerate the infusions and to give a vasoconstrictor (neosynephrine or noradrenaline) to counteract this effect.
- Pulmonary hypertension; the heparin-protamine complex triggers the release of thromboxane A2 , which is a pulmonary vasoconstrictor; PAP rises by 25-50%.
- Antigen-antibody reaction (IgG and IgE) (type II reaction); rarer, this occurs in diabetics treated with insulin stabilised with protamine (protamine-Zn), in re-operated patients who have previously received protamine, in fish-allergic patients, and in vasectomized men.
- Anaphylactoid reaction triggered by the heparin-protamine complex by directly activating the classical complement pathway (C4a) without the intermediary of an antigen-antibody reaction (type III reaction, which occurs in 1.5% of cases). The clinical picture is that of a major anaphylactic reaction with severe hypotension; the massive release of thomboxane triggers pulmonary hypertension and bronchoconstriction which can be life-threatening. Treatment includes hydrocortisone, prostaglandin E1 and vasoconstrictors (noradrenaline, vasopressin, methylene blue).
- In the absence of heparin, or in excess of heparin (protamine/heparin ratio > 1.3:1), protamine has an anticoagulant effect by three different mechanisms. It causes thrombocytopenia and a decrease in platelet aggregability of about 50%; it inhibits the activation of factors V and VII; it induces fibrinolysis [2]. Blood loss increases by 26% when the heparin:protamine ratio is 1.3 instead of 0.8 [9].
Patients are sensitised to protamine by previous cardiovascular surgery or by insulin containing protamine as a stabiliser; patients allergic to fish protein are likely to develop a violent reaction. Patients at risk can be anticoagulated with bivalirudin, whose effect is not reversed by protamine, or operated on with pre-heparinised bypass circuits and reduced systemic heparinisation (100 IU/kg), which may avoid the need for protamine [10]. A steroid dose during ECC (500 mg methylprednisolone) and a very slow injection (infusion over 20 minutes) via a peripheral route may decrease the intensity of reaction in an allergic patient.
Pentasaccharides: fondaparinux (Arixtra® )
Pentasaccharides are synthetic analogues of the saccharide sequence that binds heparin to AT III (see Figure 8.10). They correspond to the high-affinity segment, which is the minimum chain length required to induce conformational change, without having any bridging ability. Thus, unable to inhibit factor IIa (thrombin), they selectively inhibit factor Xa [15]. These agents do not bind to other proteins or cell membranes, and therefore have linear pharmacokinetics and a well-predictable dose-response curve. They do not require special monitoring, but require subcutaneous administration.
Fondaparinux (Arixtra® ) has a half-life of 16-17 hours and is not metabolised but is completely excreted by kidneys. It should be avoided in case of renal failure (creatinine clearance < 20 mL/min), reduced to 1.5 mg/d if clearance is 20-30 mL/min and halved if clearance is 30-50 mL/min [4]. It is indicated for prevention of thromboembolic events. The target value for a prophylactic plasma concentration is 0.2-0.7 mcg/mL. The effect is measured by the calibrated anti-Xa activity for the substance, but is not routinely required. The dosage is 7.5 mg once daily subcutaneously. In obese patients (> 100 kg), it is increased to 10 mg/d [5]. Protamine does not reverse the effect of fondaparinux, which has no specific antagonist (see Antagonism).
Idraparinux is an analogue of fondaparinux with a long half-life (130 hours) and only requires one subcutaneous injection per week. The addition of biotin to the molecule (idrabiotaparinux) allows the action of an antagonist, avidin, which binds to biotin [15].
| Indirect anti-thrombin agents |
| Heparin binds to antithrombin III (AT III) and accelerates its ability to inhibit factors IIa (thrombin) and Xa 2,000-fold. Its action depends on a sufficient amount of AT III. Unfractionated heparin (UFH) has an anti-Xa / anti-IIa activity ratio of 1:1. Low molecular weight heparins (LMWH) have an anti-Xa / anti-IIa ratio of 2-4:1; fondaparinux selectively inhibits factor Xa.
UFH binds to other proteins and its activity is highly variable, hence the need for monitoring by aPTT and ACT. By the iv route, its effect is immediate, but its biological half-life (1-2.5 hours) is dose dependent. Elimination through the reticuloendothelial system. UFH does not inhibit fibrin-bound thrombin in a thrombus. Antagonist: protamine; 1 mg protamine (100 IU) neutralizes 1 mg heparin (100 IU). Risk of thrombocytopenia (HIT): 1-5%. Preoperative infusion interruption time: 4 hours. Dosages iv :
- Acute thromboembolism: bolus 80 IU/kg (5,000 IU), maintenance 18 IU/kg/h (32,000 IU/24h)
- Acute coronary syndrome: bolus 60-70 IU/kg, maintenance 12-15 IU/kg/h
- ECC: 300-400 IU/kg iv
LMWH (Clexane® , Lovenox® , Fraxiparine® , etc) have more of an anti-Xa effect than an anti-thrombin effect. Their half-life varies from 4 to 7 hours, but is extended to 17 hours when creatinine clearance is < 50 mL/min. In renal failure, doses should be halved or withheld. Protamine is a partial antagonist. Subcutaneous dosages:
- Prophylactic: 5,000-10,000 IU/day; preoperative withdrawal time: 12 hours
- Treatment: 20,000 IU/d (250 IU/kg/d); preoperative withdrawal time: 24 hours
Fondaparinux (Arixtra® ) selectively inhibits factor Xa. It has a half-life of 16-17 hours and is excreted by kidneys. In case of renal insufficiency, doses should be reduced by half or withheld. Subcutaneous dosage: 7.5 mg/day. No antagonist. Preoperative withdrawal time: 48-72 hours.
Protamine: reverses the effect of UFH in a 1mg to 1mg ratio. Usual dose: 80% of UFH dose in slow iv (10 minutes). Side effects: hypotension, AG-AC reaction, anaphylactoid shock with pulmonary hypertension and vasoplagia; in overdose (UFH/protamine ratio > 1.3:1), has an anticoagulant effect.
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- ADAMS RLC, BIRD RJ. Review article: Coagulation cascade and therapeutic update: Relevance to nephrology. Part I: Overview of coagulation, thrombophilia and history of anticoagulants. Nephrol 2009; 14:462-70
- BOER C, MEESTERS MI, VEERHOEK, VONK ABA. Anticoagulant and side-effects of protamine in cardiac surgery: a narrative review. Br J Anaesth 2018; 120:914-27
- FINLEY A, GREENBERG C. Heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg 2013; 116:1210-22
- GARCIA DA, BAGLIN TP, WEITZ JI, et al. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e24S-e43S
- HOLBROOK A, SCHULMAN S, WITT DM, et al. Evidence-based management of anticoagulant therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e152S-e184S
- KIMMEL SE, SEKERES M, BERLIN JA, et al. Mortality and adverse events aTFer protamine administration in patients undergoing cardiopulmonary bypass. Anesth Analg 2002; 94:1402-8
- LOWENSTEIN E, ZAPOL WM. Protamine reactions, explosive mediator release and pulmonary vasoconstriction. Anesthesiology 1990; 73:373-8
- MANSON L, WEITZ JI, PODOR TJ, et al. The variable anticoagulant response to unfractionated heparin in vivo reflects binding to plasma proteins rather than clearance. J Lab Clin Med 1997; 130:649-655
- MEESTERS MI, VEERHOEK D, DE LANGE F, et al. Effect of high or low protamine dosing on postoperative bleeding following heparin anticoagulation in cardiac surgery. Thromb Haemost 2016; 116:251-61
- MUKADAM ME, PRITCHARD D, RIDDINGTON D, et al. Case 7-2001. Management during cardiopulmonary bypass of patients with presumed fish allergy. J Cardiothorac Vasc Anesth 2001; 15:512-9
- NUTESCU EA, SPINLER SA, WITTKOWSKY A, et al. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother 2009; 43:1064-83
- RANUCCI M, CAZZANIGA A, SORO G, et al. The anti-thrombin III saving effect of reduced systemic heparinization and heparin-coated circuits. J Cardiothorac Vasc Anesth 2002; 16:316-20
- ROSEN D, ROSEN K. Elimination of drugs and toxins during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997; 11:337-40
- ROZENTA T, SHORE-LESSERSON L. Pharmacologic management of coagulopathy in cardiac surgery: An update. J Cardiothorac Vasc Anesth 2012; 26:660-79
- SINAURIDZE EI, PANTELEEV MA, ATAULLAKHANOV FI. Anticoagulant therapy: basic principles, classic approaches and recent developments. Blood Coag Fibrinol 2012; 23:482-93
- WEITZ JI, HIRSH J. New anticoagulant drugs. Chest 2001; 119:95s-107s