The vast majority of cases of tricuspid regurgitation (TI) are not related to valve pathology, but are secondary to dilatation of the right ventricle and tricuspid annulus.
Video: Moderate tricuspid insufficiency on RV dilatation and dysfunction in pulmonary hypertension.
- Left-sided valvular disease or LV failure leading to post-capillary pulmonary hypertension;
- Pulmonary hypertension, whatever the cause;
- LV dysfunction (volume overload, cardiomyopathy, ischaemia or infarction).
This functional TI is classified into three levels of importance [11].
- Grade I: modest annular dilatation, competent tricuspid valve or mild TI;
- Grade II: significant annulus dilatation and tricuspid coaptation defect; at least moderate TI;
- Grade III: significant dilatation of the annulus and RV, restrictive insufficiency due to excessive tension on the cords; severe TI.
While functional MI is mainly due to traction on the cords caused by LV dilatation, functional TI is more related to annulus dilatation [13].
Primary lesion of the tricuspid leaflets is a rarer condition (25% of operated cases) with a variety of aetiologies (Figure 11.153 and Figure 11.154) [1,8,12].
- Ebstein's disease: the posterior and septal leaflets are low and end in an intraventricular position; the cavity of the RV is narrowed and its entire basal part is "atrialised". The anterior leaflet, usually normally inserted, may be insufficient to allow occlusion or redundant and tilted into the RA.; in both cases the TI is significant.
Video: Ebstein's disease; the septal leaflet of the tricuspid is low-inserted, the anterior leaflet is normally inserted and large, but insufficient to occlude the valve; major IT has caused dilatation of the RV by volume overload.
- ARF: still common in non-industrialised countries, the disease results in a combination of insufficiency and stenosis due to commissural fusion, narrowing and fibrosis of the leaflets and chordae.
Video: Major tricuspid insufficiency in the setting of tricuspid disease on ARF; the RV is small but the RA dilated; the same is true on the left (mitral stenosis with small LV and dilated LA).
- Myxoid degeneration (Barlow) and flail leaflet: usually associated with mitral prolapse.
Video: Tilting of the posterior leaflet leading to severe insufficiency.
Video: Massive tricuspid insufficiency on color flow in the same case.
- Endomyocardial fibroelastosis: retraction of the leaflets and chords, common in tropical Africa.
- Trauma: column fracture or leaflet tear. In thoracic trauma, the tricuspid and free walls of the RV are the most anterior parts of the heart and therefore most exposed to shock. Closed chest trauma is common in the history of patients presenting with isolated TI [4].
- Endocarditis: the incidence is particularly high in drug addicts and people with implanted pacemakers.
- Carcinoid syndrome: Deposition of fibrous plaques, thickening and narrowing of the lamellae (see Carcinoid syndrome under Principles of Anaesthesia).
Video: Tricuspid insufficiency and stenosis in carcinoid syndrome.
- Iatrogenicity: A pacemaker-defibrillator probe or pulmonary artery catheter prevents leaflet closure and causes benign TI.
- Drug toxicity: methysergide, ergot, fenfluramine, phentermine, aminorex, benfluorex, pergolide.
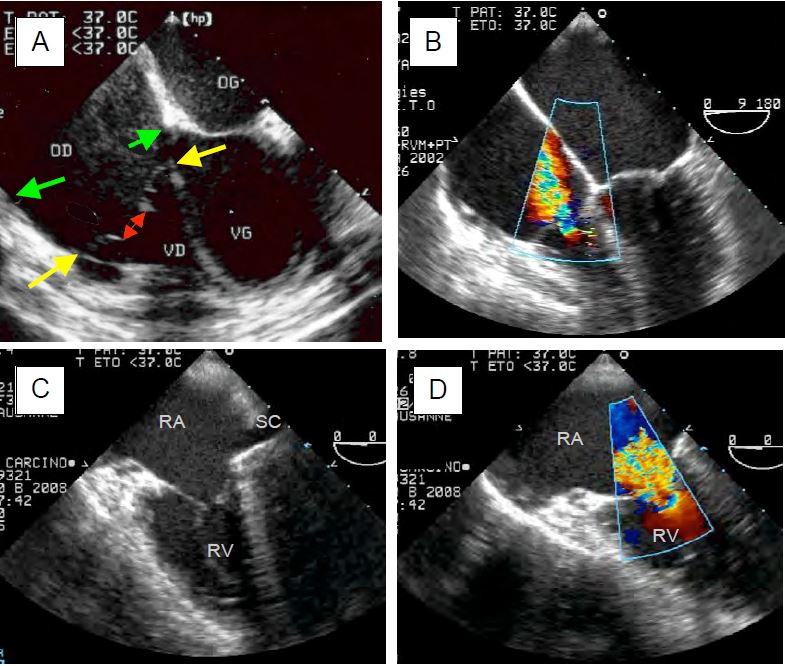
Figure 11.153: TEE images of tricuspid insufficiency (TI) of different aetiologies. A: Ebstein's disease; the septal and posterior leaflets of the valve are low in the ventricular cavity (yellow arrows); their normal insertion would be at the level indicated by the green arrows. The volume between the yellow and green arrows is the zone of atrialisation of the RV. The leaflets do not meet in systole (double red arrow), resulting in a severe TI. B: Moderate to severe tachycardia in the ARF on 0° 4-cavity view. C: 4-cavity view (low esophageal position) of massive tricuspid insufficiency in carcinoid syndrome; the leaflets are thickened, restrictive and do not coapt in systole. SC: coronary sinus. D: Identical view with colour Doppler flow; the vena contracta is large (> 1 cm); there is a significant PISA.
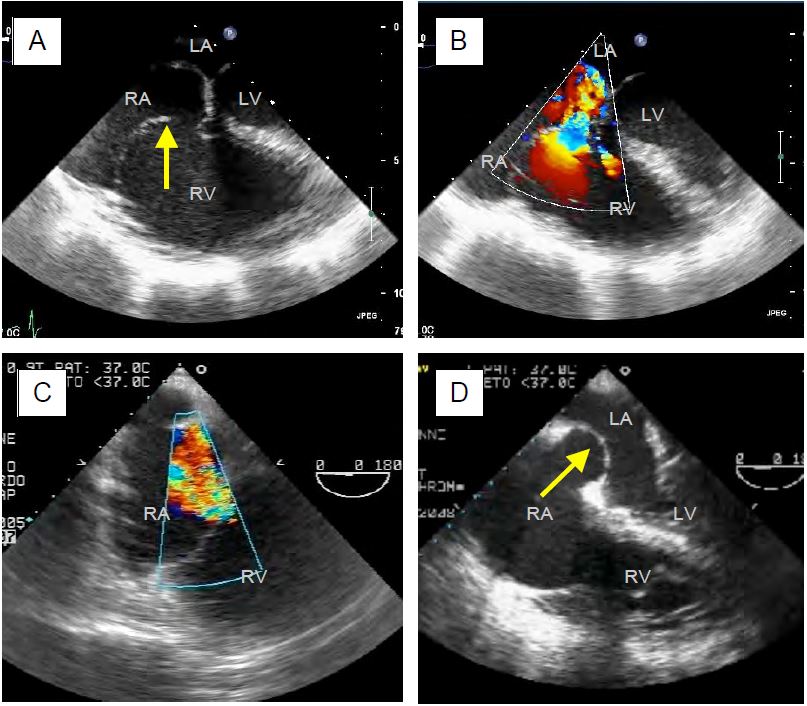
Figure 11.154: TEE images of tricuspid insufficiency (TI) of different aetiologies. A: Traumatic rupture of the chordae with tilting of the anterior leaflet. B: Same case with the massive jet of TI directed towards the interatrial septum. C: TI in pulmonary hypertension; RV and RA are dilated. D: Tilt of the interatrial septum, which is stretched into the LA; this is often the first thing that appears on the screen when the heart is monitored in 4-cavity and acute right heart failure occurs.
Pathophysiology
Because the right heart operates at a physiologically very low pressure, the tricuspid valve is not designed to be as tight as the mitral valve. Mild insufficiency is common and of no consequence as it causes only a small volume of regurgitation in a system that can accommodate large volume changes. It occurs in almost 75% of the population over the age of 50 [9]. Even if the lesion is larger, it is generally well tolerated by the RV, which is an excellent pump volume. In cases of excessive LV afterload (PHT, left ventricular stasis), the retrograde ejection of the TI acts as a pressure valve and reduces ventricular wall tension. In cases where the regurgitant fraction is very high (1-2% of cases), pulmonary anterograde flow may decrease. This situation is unfavourable for left ventricular preload for two reasons.
- The reduced pulmonary flow reduces the return to the LA;
- Dilatation of the RV causes the interventricular septum to bulge to the left in diastole, limiting LV filling: this is the Bernheim effect (see Figure 11.16 and Figure 11.152).
If it is large, the TI represents a volume overload for the RV, which tends to dilate, enlarging the tricuspid annulus and increasing the TI. This creates a vicious circle linked to the flexibility of the right structures. The silhouette of these structures varies according to the duration of the TI.
- Acute TI: slight dilatation of the RA and RV, tilted interatrial septum stretched into the LA, very increased CVP, massive "v" wave.
- Chronic TI: severe dilatation of the RA and RV, dilatative RVH, interventricular septal invagination into the LV, wide TI jet at its base, variable "v" wave as buffered by the dilatation of the RA.
The presence or enhancement of a TI is a good marker of right-sided decompensation or transient pulmonary hypertension: infarction or failure of the RV, pulmonary embolism, acute volume overload, administration of protamine after ECC, excessive PEEP, etc. The size of the TI is directly proportional to the pulmonary arterial resistance: it increases as the PAR increases. On TEE it is often the sudden tilting of the tight, bulging interatrial septum into the LA that first attracts attention (see Figure 11.154D above).
Video: Volume overload of the RA in tricuspid insufficiency; the RA is dilated, the interatrial septum permanently bulges into the LA.
Clinical manifestations
The clinical manifestations of TI are related to upstream systemic congestion and/or downstream aetiologies: pulmonary hypertension, mitral disease or LV insufficiency. As the right atrium is very compliant, pressure increases there only when regurgitation is massive or sudden. The "v" wave becomes prominent on the CVP curve; it is mesosystolic, whereas its peak is normally telesystolic. In this case, the CVP curve resembles that of RV: the "x" descent fades and the curve is dominated by the "v" wave, which is earlier than normal, with a very wide "y" descent [5]. The size of the "v" wave is not proportional to that of the TI because of the compliance of the RA: when it is dilated, the RA absorbs the regurgitated volume without much change in pressure (high compliance); the wave is most visible in acute cases where the compliance of the RA is normal. If the pressure in the RA becomes higher than that in the LA, a cyanogenic R - L shunt may develop as a result of reopening of the foramen ovale if it was not fused to the septum; this may also occur during Valsalva manoeuvres or controlled ventilation with high PEEP [3].
Arrhythmias are common as they are related to the dilatation of the cavity: suprajunctional tachyarrhythmias (dilatation of the RA.), ventricular tachyarrhythmias (dilatation of the RV.). The clinical status revealed systemic venous congestion, peripheral oedema, hepatomegaly (cholestatic type), ascites and pulsatility of the jugular veins. The chest x-ray showed a right-sided protrusion of the cardiac silhouette. A BNP elevation > 200 pg/ml is associated with a poor prognosis.
Echocardiography
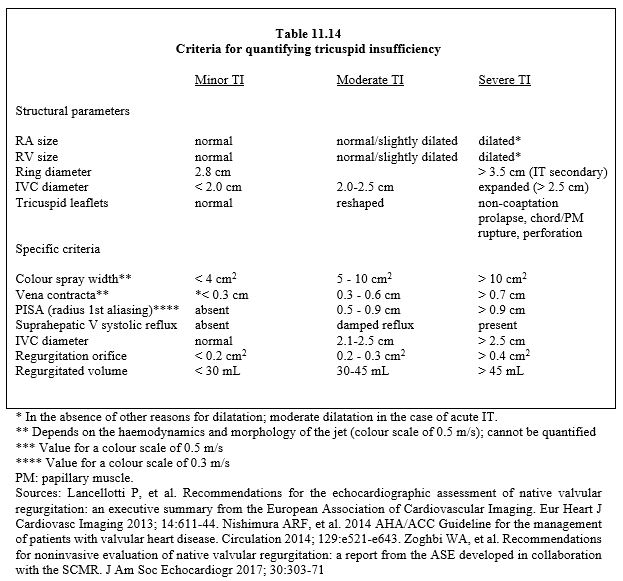
Echocardiographic images of TI are typical (see Figure 11.154 above). The extent of the colour jet is a convenient means of diagnosing the presence of TI and grossly assessing its significance, but it is not an adequate technique for accurate quantification as it depends on the force of contraction of the RV, PAR and PRA [188a]. The criteria for severe tricuspid regurgitation are essentially echocardiographic (Table 11.14) [2,6,9,15,16].
Video: Moderate tricuspid insufficiency in 60° RV inflow tract view.
- Central non-coaptation of the leaflets, which may be normal (secondary TI) or abnormal (tilting in the RA, chord rupture, retraction, perforation); leaflet distance > 8 mm.
- Dilatation of the RA (diameter ≥ 5 cm, surface area > 18 cm2); bulging of the interatrial septum into the LA.
- Dilatation of the RV and tricuspid annulus (diameter > 4.0 cm in 4-cavity view); diastolic tilting of the interventricular septum into the LV, with a "D" shaped short-axis silhouette.
- Dilatation of the coronary sinus and inferior vena cava (diameter > 2.5 cm).
- TI jet occupying more than one third of the RA or > 10 cm2, but the colour jet depends on haemodynamic conditions and is not a quantitative criterion. In the case of a large orifice and severe dysfunction of the RV, the velocity of the TI remains low and the flow laminar without aliasing, even in the case of massive insufficiency; all that is seen is a back-and-forth flow of blood between the RA and the RV (Figure 11.155).
Figure 11.155: TI jet in a case of severe LV dysfunction. The tricuspid leaflets hardly move between systole (A) and diastole (B). The jet of tricuspid regurgitation appears as a laminar retrograde flow without vortices or aliasing (red-orange) because its velocities are low due to the inability of the RV to generate sufficient pressures. Diastolic flow is normally laminar (blue).
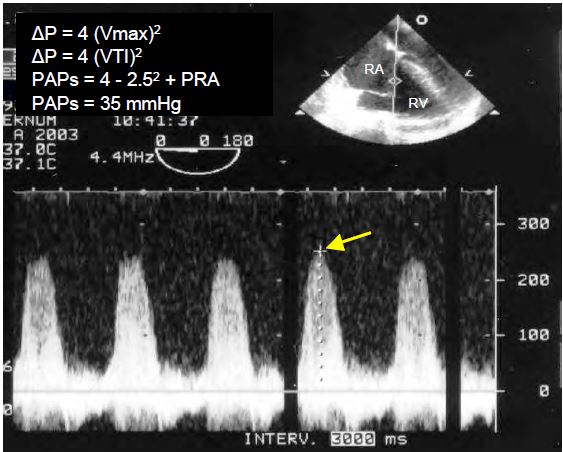
- On continuous Doppler, a spectral velocity trace that is dense and sharply contoured indicates a large TI; a triangular trace with a protosystolic peak indicates that the PRA is high (pronounced "v" wave on the CVP curve). Vmax is 2-4 m/s but depends on the function of RV and PAP; it does not give a specific indication of the size of the regurgitant volume as it is often low in massive TI.
Video: Major tricuspid insufficiency in a case of arrhythmogenic RV dysplasia; RV failure is such that systolic reflux is low-velocity, with no vortex or aliasing. On the other hand, the valve leaflets leave a large regurgitation orifice in systole.
- Accelerated diastolic anterograde flow (Vmax of E flow > 1 m/s).
- Diameter of the jet at the vena contracta > 0.7 cm; in 3D, a vena contracta area > 0.4 cm2 indicates severe TI (Figure 11.156).
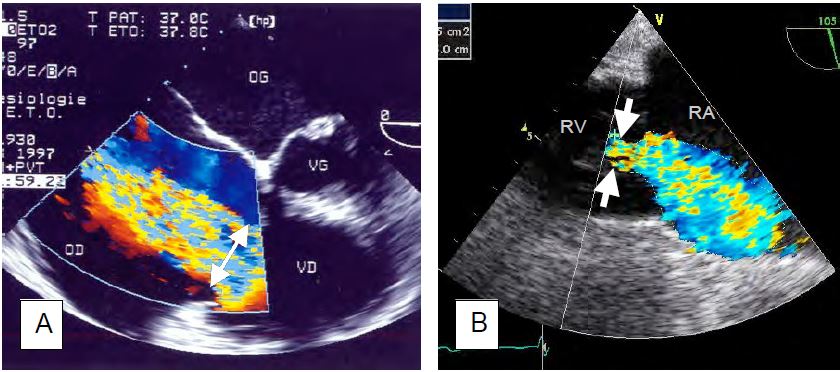
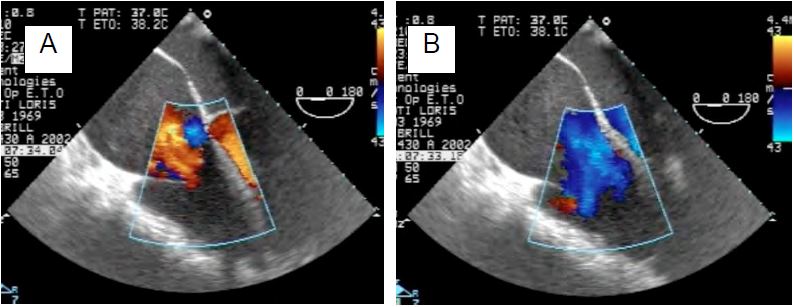
Figure 11.156: Quantification of severe tricuspid regurgitation. A: Very large vena contracta (2.2 cm) in massive TI on ARF (4-cavity view). B: Planimetry of the dye jet (11.5 cm2) and measurement of the diameter of the vena contracta (0.8 cm) in transgastric view (100° RV inlet view).
- Proximal flow convergence area (PISA): a radius of 1er aliasing > 9 mm (colour scale of 28 cm/s) indicates severe TI; this method is rarely used in TI because of the low velocity and flattened hemispheric area, which tends to underestimate the extent of regurgitation.
- Regurgitant orifice ≥ 0.4 cm2, regurgitant volume ≥ 45 mL; in 3D, the surface area of the regurgitant orifice is > 0.75 cm2. These values are not well established and remain controversial.
- Inversion of the systolic component of flow in the IVC and suprahepatic veins, which are dilated and pulsatile; the absence of this inversion does not exclude severe TI (Figure 11.157). On the other hand, venous systolic flow is influenced by PRA, compliance of the right cavities, respiration (measurements should be taken during apnoea) and rhythm disturbances (atrial fibrillation, atrioventricular block, pacemaker).
- In secondary TI, distance and tent area are > 0.75 cm and > 1.6 cm2 respectively.
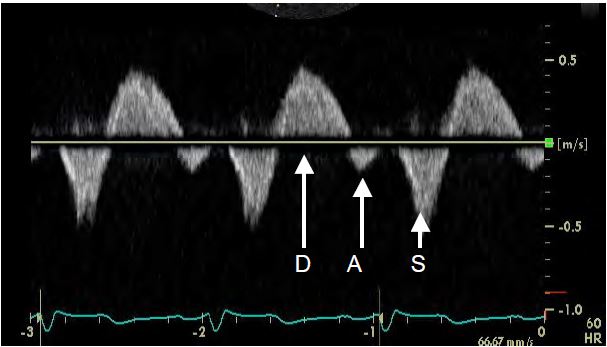
Figure 11.157: Flow in a suprahepatic vein (TEE in transgastric position). Systolic reflux (S) is seen, whereas normal flow should be anterograde. D: anterograde diastolic flow. A: Mild reflux due to atrial contraction.
- On TEE and under controlled ventilation, a diameter variation of > 20% between inspirium and expirium indicates hypovolaemia;
- In TTE and spontaneous breathing, the threshold for hypovolaemia is a respiratory variation of > 50%.
- In hypervolaemia, the IVC is dilated (diameter > 2.5 cm) and does not pulsate.
| Characteristics of tricuspid insufficiency |
| RV volume overload (well tolerated)
TI increases as PAR increases
Low tolerance of dilated RV to increase in afterload
Systemic stasis
If TI is secondary to PHT or left-sided pathology, these will dominate haemodynamics.
Primary TI (25% of cases): organic leaflet disease
Secondary TI (75% of cases): due to LV dysfunction, PHT, left-sided valve disease, LV dysfunction
Criteria for severe TI:
- Systemic congestion
- PVC ↑ ( v wave ↑ )
- RA and RV dilatation, tricuspid annulus diameter > 3.5 cm
- Bulging of the interatrial septum into the LA
- Large TI jet (vena contracta diameter > 0.7 cm)
- Regurgitant orifice ≥ 0.4 cm2, regurgitant volume > 45 mL
|
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- BRUCE CJ, CONNOLLY HM. Right-sided valve disease deserves a little more respect. Circulation 2009; 119:2726-34
- CHERRY SV, JAIN P, RODRIGUEZ-BLANCO YF, FABBRO M. Noninvasive evaluation of native valvular regurgitation: a review of the 2017 American Society of Echocardiography Guidelines for the perioperative echocardiographer. J Cardiothorac Vasc Anesth 2018; 32:811-22
- CUJEC B, POLASEK P, MAYERS I, et al. Positive end-expiratory pressure increases the right-to-left shunt in mechanically ventilated patients with patent foramen ovale. Ann Intern Med 1993; 119(9):887-94
- GAYET P, PIERRE B, DELAHAYE JP. Traumatic tricuspid insufficiency: An underdiagnosed disease. Chest 1987; 92:429-35
- GROSSMAN W, ed. Cardiac catheterization and angiography. 3rd edition. Philadelphia, Lea and Febiger, 1986, 378
- HAHN RT. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging 2016; 9:e005332
- JANDA S, SHAHIIDI N, GIN K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart 2011; 97:612-22
- KIM JB, SPEVACK DM, TUNICK PA, et al. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: an observational study. J Am Soc Echocardiogr 2008; 21:284-7
- LANCELLOTTI P, TRIBOUILLOY C, HAGENDORFF A, et al. Recommendations for the echocardiographic assessment of native valvular regurugitation: an executive summary from the EACI. Eur Heart J Cardiovasc Imaging 2013; 14:611-44
- LANG RM, BADANO LP, TSANG W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012; 13:1-46
- RAJA SG, DREYFUS GD. Basis for intervention on functional tricuspid regurgitation. Semin Thorac Cardiovasc Surg 2010; 22:79-83
- ROGERS JH, BOLLING SF. The tricuspid valve. Current perspective and evolving management of tricuspid regurgitation. Circulation 2009; 119:2718-25
- TARAMASSO M, VANERMEN H, MAISANO F,et al. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol 2012; 59:703-10
- THUNBERG CA, GAITAN BD, GREWAL A, et al. Pulmonary hypertension in patients undergoing cardiac surgery: Pathophysiology, perioperative management, and outcomes. J Cardiothorac Vasc Anesth 2013; 27:551.72
- TRIBOUILLOY C, ENRIQUEZ-SARANO M, BAILEY MR, et al. Quantification of tricuspid regurgitation by measuring the width of the vena contracta with Doppler color flow imaging: a clinical study. J Am Coll Cardiol 2000; 36:472-8
- ZOGHBI WA, ADAMS D, BONOW RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the ASE developed in collaboration with the SCMR. J Am Soc Echocardiogr 2017; 30:303-71