In order to better manage haemodynamics and reduce inflammatory response, the concept of "Mini-CEC" or MiECC (Minimally invasive Extra-Corporeal Circulation) has recently been created, which aims to reduce the priming volume of the circuit and eliminate the air-blood contact surface. To achieve it, the length of the tubing is reduced (< 1 m) and the cardiotomy reservoir is eliminated. The pump is a centrifugal one that is used for both venous drainage by suction and propulsion of blood back to the systemic arterial side ; it is sensitive to preload and afterload. The system operates at constant volume. In addition the circuit includes a membrane oxygenator, a heat exchanger and a bubble filter on the venous side (Figure 7.16). The arterial flow is directly coupled to the venous return; the patient's central capacitance is the venous reservoir, so the CVP must remain between 3-8 mmHg. Blood drawn from the surgical field should be processed by CellSaver™ before being returned to the patient. In case of valvular surgery or complex cases, the lack of reservoir is compensated for by a system that allows cardiotomy blood to be drawn into a flexible reservoir or rigid vacuum-regulated tray, which is external to the system and acts as an expansion vessel placed in a bypass on the venous line [7]. Several systems are currently on the market: Jostra's MECC™ (450 mL priming), Dideco's ECC.O™ (< 600 mL priming), Sorin Biomedica's IDEAL™ (900 mL priming), etc. The priming volume can be further reduced by performing a partial retrograde filling once the patient is cannulated: 150-200 mL of blood is drawn through the aortic cannula immediately after the ECC cannulation.
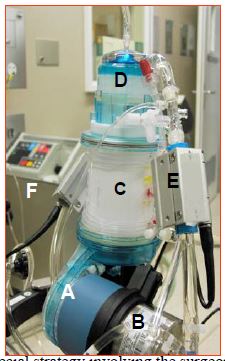
Figure 7.16: Mini-CEC (Dideco's ECC.O™ device). A: non-occlusive centrifugal pump. B: heat exchanger. C: oxygenator. D: arterial filter. E: bubble and arterial saturation monitor on the arterial circuit. F: external water cooling and heating system for the heat exchanger.
The mini-CEC is not only a smaller circuit but a dedicated strategy involving the surgeon, the anaesthetist and the perfusionist. It will involve [5]:
- Closed ECC system with limited volume.
- Biocompatible (heparin-coated) surface of the components in contact with blood.
- Reduced priming volume (< 750 mL).
- Bubble trap on the venous circuit.
- Centrifugal pump (provides both venous drainage and arterial propulsion); different models exist on the market: BioMedicus™, CentriMag™, RotaFlow™, Capiox™.
- Membrane oxygenator (micropore); the miniaturisation of the oxygenator reduces its exchange surface, forcing a Hb ≥ 10% higher than in conventional circuits. A heat exchanger coupled to the oxygenator.
- Independent cardioplegia circuit.
- Cardiotomy suction with blood washing, independent of the circuit.
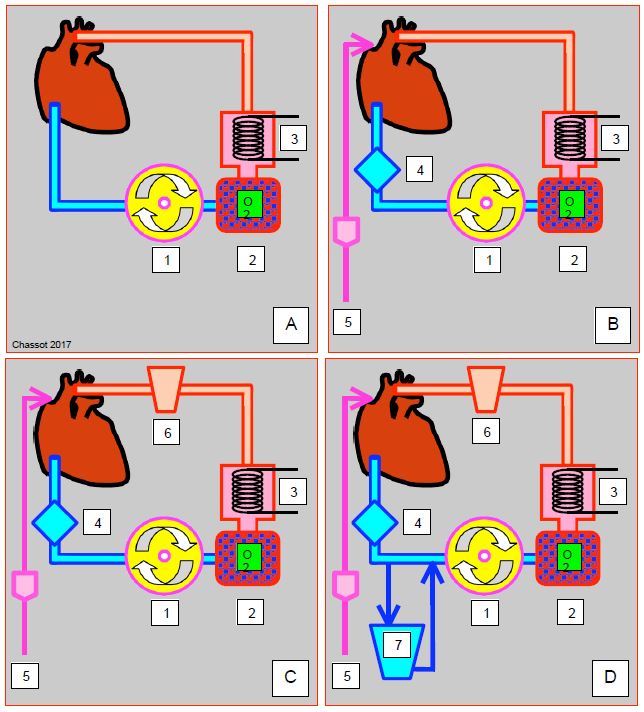
The system displays several degrees of complexity (Figure 7.17) [1].
- Type I: venous drainage of the RA® pump® oxygenator/heat exchanger® arterial cannula with filter; this is the simplest system, but it is not protected against the risk of air being sucked in through venous cannulae.
- Type II: same, with the addition of a bubble trap on the venous return, and the possibility of suctioning and recovering blood from cardiotomy drains (LV, pulmonary vein, aorta); blood is aspirated, washed and returned in an independent circuit.
- Type III: Same as type II, with the addition of a flexible reservoir (without blood-air contact) to store and reperfuse blood.
- Type IV: Same as Type III, with an external rigid reservoir mounted on the venous return; in this case, the closed system becomes a semi-open system.
Figure 7.17: Schematic representation of the various mini-CEC set-ups. A: Minimal system (type I); venous drainage from the RA into the pump (1) which propels the blood into the oxygenator (2) and heat exchanger (3) and then into the aorta. B: Type II; a bubble trap is added to the venous ciruit (4); the cardiotomy suction (5) is drained, washed and returned to an independent circuit. C: Type III; a collapsible reservoir (6) is used to collect blood from the aspirations. D: Type IV; the addition of a rigid venous reservoir (7) bypassing the RA return creates a semi-open circuit similar to a conventional circuit [1].
Although more challenging to manage than a standard circuit, MiECC has a number of biological and clinical advantages [5].
- Reduced haemodilution: Ht is 25% higher when coming off bypass; the transfusion rate is reduced by 50-75%, making it particularly suitable for patients who refuse transfusions [4,15,17,19].
- Less heparinisation (150 IU/kg): the desired ACT is 300-350 sec for coronary artery surgery and 400-450 sec for valve surgery [9].
- Decreased coagulation alterations due to biocompatible circuits, low haemodilution and non-occlusive centrifugal pump; post-ECC platelet count is 25% higher [15,19].
- Reduced inflammatory response due to preheparinised and biocompatible circuits, reduced plastic-blood contact area, and no blood-air contact. SIRS markers are lowered [6,10].
- Better adaptation to fast-track anaesthetic techniques [3].
Other advantages are mentioned by the proponents of the MiECC, although they are controversial and not unanimously supported in the literature.
- Higher MAP, better splanchnic perfusion [17].
- (Doubtful) reduction in AF incidence by 50% [2,6].
- Preservation of renal function; postoperative creatinine increase is 20% lower [11,15].
- Improved myocardial protection; postoperative CK MB levels are reduced and the need for catecholamine is halved [12,15].
- Decreased gaseous microemboli: decreased neurocognitive impairment (OR 0.30-0.56) [8,14,19].
- Functional improvement of target organs (lung, liver, intestine), lowered lactate levels [19].
- Reduced mortality in coronary artery ECC in some studies [2,16].
With this type of circuit, it is good that patients are relatively hypervolaemic at the time of connection, since the patient is the venous reservoir of the bypass machine. The patient's venous capacity is therefore critical for haemodynamic stability. This is ensured by position (leg elevation, Trendelenburg/anti-Trendelenburg) and by vasoactive agents. (phenylephrine 100 mcg, nitroglycerin 25-50 mcg). For cannulation and decanulation or for aortic clamping, MAP is additionally lowered by venous return restraint (surgical compression) or by rapid pacing. The system does not present any problems with postoperative balance because the water supply is much lower than with conventional circuits. During weaning, venous clamping is progressive as is the reduction in pump output. These systems are particularly well suited to beating heart circulatory support, which combines the absence of cardioplegia with haemodynamic support from a heart-lung machine, thus eliminating the risks of cardiac arrest and aortic clamping while retaining the advantages of a pump for performing delicate coronary anastomoses.
With these systems, there is a perfect match between the blood drained and the volume reinfused by the pump. Thus, in case of low venous return or accidental air intake (about 50 mL), the system stops. Conversely, an increase in venous return requires an acceleration of the pump flow. Operating ECC is therefore more tricky, the pump flow rate is constantly adjusted. The anaesthetist must ensure that there are no air bubbles introduced through the venous lines: closed valves, caps on injection sites, drugs administered preferably as an infusion rather than a bolus, curarisation to avoid respiratory movements, etc. When the technique is well mastered and coordination between the surgeon, anaesthetist and perfusionist is satisfactory, haemodynamics are very stable and perfusion pressure is better maintained than with traditional systems. High risk cases are the main beneficiaries.
For the anaesthetist, this type of circuit has several practical implications.
- Constant adjustment of pressure and flow with vasopressors or vasodilators to maintain preload of the bypass; positional changes.
- Fast-track technique: reduced total opioid dose, dexmedetomidine infusion.
- Intravenous (propofol infusion) or inhalation (halogen spray on fresh gas supply) anaesthesia.
- Maintain normal to high preload prior to bypass (infuse 500 mL crystalloid + 500 mL colloid pre-pump).
- Managing the return of aspirations through the CellSaver™.
- Very restrictive indications for transfusion.
Despite the benefits described in the most favourable publications, MiECC has not become a routine technique, as it undeniably presents a more tense management and a lower margin of manoeuvre than conventional ECC. Furthermore, it is not suitable for all types of surgery.
Port-access™ system (HeartPort)
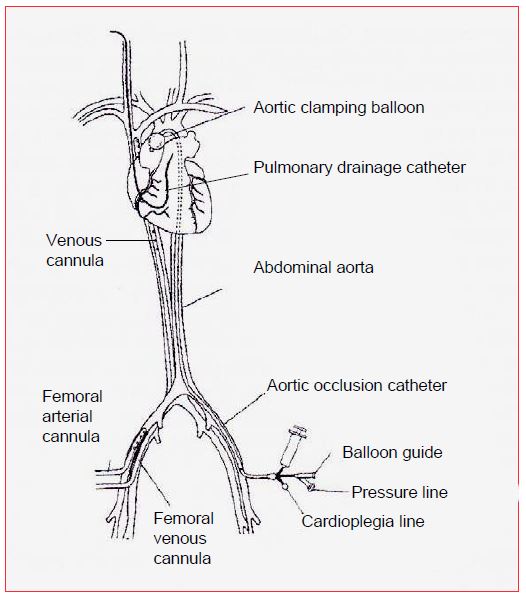
This system aims to perform cardiac surgery in a minimally invasive manner, providing all the ECC cannulation strictly percutaneously. It consists in several components (Figure 7.18) [13,18].
Figure 7.18: Port-access™ cannulation system (HeartPort, USA). All ECC cannulas are inserted percutaneously. The arterial cannula is in the femoral position. The venous cannula is ascended from the femoral vein into the RA. Aortic clamping is performed by a balloon mounted on a catheter and inflated in the ascending aorta; this catheter is also used to administer cardioplegia. LV drainage is provided by a catheter positioned in the PA trunk from the right internal jugular vein. The coronary sinus is cannulated through the right internal jugular vein.
- Arterial bypass cannula in the femoral artery;
- ECC venous cannula into the femoral vein and up into the RA;
- A catheter inserted through the femoral artery and raised into the root of the aorta; this catheter has an occlusive balloon that is inflated in the ascending aorta (equivalent to aortic clamping); its distal end allows for anterograde administration of cardioplegia and measurement of the pressure in the root of the aorta
- Drainage catheter inserted through the right internal jugular vein into the PA trunk;
- Retrograde cardioplegia catheter (coronary sinus) introduced through the right internal jugular vein.
The positioning of the catheters is performed under TEE control. The position of the aortic clamping balloon must be constantly monitored, as it moves easily: if it moves forward, it will impact on the aortic valve and create an acute insufficiency; if it moves back, it will block the vessels of the aortic arch and occlude cerebral perfusion. A right radial arterial catheter and cerebral saturations (ScO2 ) allow the flow in the brachiocephalic trunk to be controlled. After initial enthusiasm, this technique is struggling to find its place, except in robotic surgery. It is true that it is complicated and costly, that it extends the duration of the operation and that it carries its own risks. Its only interest is to allow a real non-invasiveness within the framework of a surgery by thoracoscopy or mini-incisions.
| Mini-CEC |
| Miniaturisation of the circuits and elimination of the cardiotomy reservoir minimises contact of the blood with foreign surfaces and eliminates contact with air, thereby inhibiting the release of inflammatory triggers. The small system volume (600-900 mL) and autologous priming reduce haemodilution. However, the lack of a compliance reservoir between the patient and the bypass makes management more difficult and not applicable to all types of surgery. |
© CHASSOT PG, GRONCHI F, April 2008, last update December 2019
References
- ANASTASIADIS K, ANTONITSIS P, ARGIRIADOU H, et al. Modular minimally invasive extracorporeal circulation systems; can they become the standard practice for performing cardiac surgery? Perfusion 2015; 30:195-200
- ANASTASIADIS K, ANTONITSIS P, HAIDICH AB, et al. Use of minimal extracorporeal circulation improves outcome after heart surgery; a systematic review and meta-analysis of randomized control trials. Int J Cardiol 2013; 164:158-69
- ANASTASIADIS K, ASTERIOU C, ANTONITSIS P, et al. Enhanced recovery after elective coronary revascularisation surgery with minimal versus conventional extracorporeal circulation: a prospective randomized study. J Cardiothorac Vasc Anesth 2013; 27:859-64
- ANASTASIADIS K, ASTERIOU C, DELIOPOULOS A, et al. Haematological effects of minimal compared to conventional extracorporeal circulation after coronary revascularisation procedures. Perfusion 2010; 25:197-203
- ANASTASIADIS K, MURKIN J, ANTONITSIS P, et al. Use of minimal invasive extracorporeal circulation in cardiac surgery: principles, definitions and potential benefits. A position paper from the Minimal invasive Extra-Corporeal Technologies international Society (MiECTiS). Interact Cardiovasc Thorac Surg 2016; 22:647-62
- BIANCARI F, RIMPILAINEN R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart 2009; 95:964-9
- CALDERON J, SIGONNEY P, JANVIER G. MECC: minimal extra-corporeal circulation. In: JANVIER G, LEHOT JJ (ed). Circulation extracorporelle: principes et pratique, 2nd edition. Paris, Arnette Groupe Liaison SA, 2004, pp 541-7
- EL-ESSAWI A, HAJEK T, SKORPIL J, et al. Are minimized perfusion circuits the better heart-lung machines? Final results of a prospective randomized multicentre study. Perfusion 2011; 26:470-8
- FROMES Y, DAGHIDJIAN K, CAUMARTIN L, et al. A comparison of low vs conventional dose heparin for minimal cardiopulmonary bypass in coronary artery bypass grafting surgery. Anaesthesia 2011; 66:488-92
- FROMES Y, GAILLARD D, PONZIO O, et al. Reduction of the inflammatory response following coronary bypass grafting with total minimal extracorporeal circulation. Eur J Cardiothorac Surg 2002; 22:527-33
- HUYBREGTS RA, MORARIU AM, RAKHORST G, et al. Attenuated renal and intestinal injury after use of a mini-cardiopulmonary bypass system Ann Thorac Surg 2007; 83:1760-6
- NGUYEN BA, SULEIMAN MS, ANDERSON JR, et al. Metabolic derangement and cardiac injury early after reperfusion following intermittent cross-clamp fibrillation in patients undergoing coronary artery bypass graft surgery using conventional or miniaturized cardiopulmonary bypass. Mol Cell Biochem 2014; 395:167-75
- PREISMAN S, KEIDAN I, PEREL A, et al. Anesthesia for port-access cardiac surgery in a pediatric population. J Cardiothorac Vasc Anesth 2005; 19:526-9
- REINEKE D, WINKLER B, KONIG T, et al. Minimized extracorporeal circulation does not impair cognitive brain function after coronary artery bypass grafting. Interact CardioVasc Thorac Surg 2015; 20:68-73
- REMADI JP, RAKOTOARIVELO Z, MARTICHO P, et al. Prospective randomized study comparing coronary artery bypass grafting with the new mini-extracoproreal circulation Jostra System or with standard cardiopulmonary bypass. Am Heart J 2006; 151:198.e1-198.e7
- RIED M, HANEYA A, KOLAT P, et al. Emergency coronary artery bypass grafting using minimized versus standard extracorporeal circulation - a propensity score analysis. J Cardiothorac Surg 2013; 8:59
- SAKWA MP, EMERY RW, SHANNON FL, et al. Coronary artery bypass grafting with a minimized cardiopulmonary bypass circuit: a prospective randomized trial. J Thorac Cardiovasc Surg 2009; 137:481
- VISTARINI N, AIELLO M; MATTIUCCI G, et al. Port-access minimally invasive surgery for atrial septal defects: a 10-year single-center experience in 166 patients. J Thorac Cardiovasc Surg 2010; 139:139-45
- ZANGRILLO A, GAROZZO FA, BIONDI-ZOCCAI G, et al. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: a meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg 2010; 139:1162-9