Halogens are widely used in ECC, but their kinetics are significantly altered in this situation. The rate of increase in gas concentration is slower in hypothermic ECC because of the increase in cold solubility; however, gas extraction is as rapid as under standard conditions. For desflurane, for example, half of the inspired concentration (6%) is reached in 5 minutes and then peaks at 68% after 32 minutes [12]. For the clinician, this means that the usual gas concentrations are not achieved during hypothermic ECC. To achieve the desired effect, the inspired concentration must be increased and more time must be considered. On average 5-10 minutes should be elapsed before the full haemodynamic effect of the halogen is achieved. If the halogen is the anaesthetic agent used prior to ECC, its concentration will decrease as the pump starts and cools, so its concentration on the oxygenator must be increased relatively to the anaesthesia machine. More over is the effect of haemodilution, which decreases the solubility of the halogens but increases their volume of distribution. Fortunately, the body's requirements decrease with cold. Thus the MAC of isoflurane decreases by 5% per degree of temperature down to 20°C [1]. Being fat-soluble, halogens are also absorbed into plastics and oxygenators; some models have a capacity for isoflurane equivalent to 17 litres of blood [16]. There are several problems with the administration of halogens in the bypass circuit, because manufacturers have never incorporated a vaporiser in their machines [3].
- The type of oxygenator is important. Microporous polypropylene membrane oxygenators, the most commonly used, partially absorb halogens but do not interfere with their diffusion. Polymethylpentene membrane oxygenators, on the other hand, block the passage of halogens; they are mainly used in long-term circuits such as ECMO, but rarely in the operating room [17].
- In their liquid form, halogens cause considerable damage to the plastics of the ECC. The vaporizerer should therefore always be placed at the bottom of the machine.
- To avoid polluting the operating theatre, halogens must be separately eliminated from the oxygenator gas outlet. This is where their exhaled fraction (Fe) should be measured, which correlates closely with the actual MAC received by the patient, whereas the inspired fraction (Fi) displayed on the vaporizer does not; but this measurement is not routine and presents some technical difficulties.
- Tolerance of home-made vaporizer setup on the ECC machine varies from country to country. While this is well accepted in North America, the attitude is more restrictive in the European Union, where a directive requires any modification or addition to the ECC circuit to be officially approved and certified as safe.
If a predetermined concentration of an intravenous agent is sought by continuous infusion (TCI: target concentration infusion), haemodilution, increased volume of distribution, decreased clearance and sequestration in the bypass machine and lungs reduce the expected effect in relation to the administered dose. The elimination half-life is prolonged. For propofol, the concentration change is 10-20% [5]. For midazolam and sufentanil, the concentrations obtained are respectively 13% and 42% lower than the calculated concentrations [4]. The half-life of midazolam after ECG is significantly prolonged [11]. The place of injection is important; for a centrally injected tracer, it takes 5 minutes for half the dose to appear in the arterial tract. If injected directly into the oxygenator inlet, 95% of the dose is delivered in a single circulatory time.
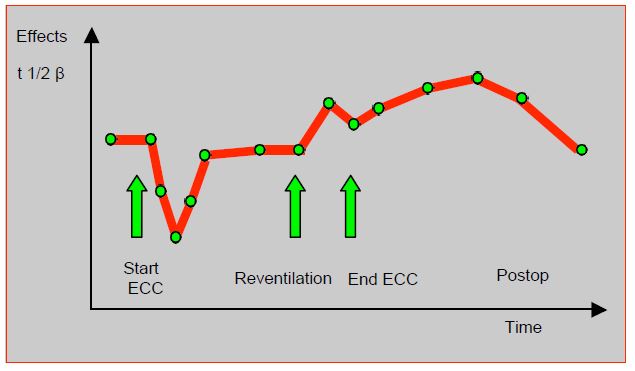
For opiates, there is a sharp but brief reduction in circulating concentrations at the start of ECC (fentanyl 53%, sufentanil 34%), followed by rebalancing of levels by redistribution from the tissues [6,8,14]. Opioid adsorption from the bypass circuitry only contributes to the initial drop, but is of no further clinical significance. It is proportional to the lipid solubility of the agents: sufentanil > fentanyl > alfentanil > remifentanil [15]. Concentrations increase on decompression and pulmonary reperfusion, as the non-perfused lungs, which had sequestered fentanils, release them on recirculation. The elimination half-life is extended by 25% after bypass surgery, because the total volume of distribution is enlarged and clearance is decreased (Figure 7.31).
Figure 7.31: Schematic representation of changes in plasma fentanil levels during bypass surgery [4].
Hypothermia affects the affinity and binding of curares to the neuromuscular plate and decreases the hepatic clearance of vecuronium. This results in a reduction of more than 80% in the perfusion rate required to maintain blocking in hypothermic ECC, once the initial dilution episode has passed [18]. Plasma esterases are diluted in ECC and partially inhibited by cold. The clearance of atracurium, cisatracurium, mivacurium, esmolol and remifentanil, all of which are metabolised by plasma esterases, is therefore significantly lowered. For atracurium, the Hoffman reaction is decreased by 43% at 28° [9]. The hepatic rhodanases responsible for the biotransformation of nitroprusside have such a low activity that the risk of thiocyanate intoxication is high [13]. The dose of xylocaine administered during ECC should be increased from 1.5 to 2.5 mg/kg to ensure usual therapeutic levels [10]. A 36% drop in concentration of nitroglycerin is recorded during ECC due to the high uptake of nitroglycerin by the circuits [7]. Milrinone uptake on the bypass is 20% [2].
| Pharmacology in ECC |
|
Because of haemodilution and temperature variations, ECC has a major influence on the pharmacokinetics of anaesthetic agents. These effects are variable depending on substance and temperature; clinical translation is the result of these various changes.
↓ sudden concentration at the beginning of the ECC (risk of awakening and decurarisation)
↑ volume of distribution
↓ concentration by sequestration (lungs, ECC circuits)
↑ effective concentration and duration of action by: ↓ clearance, ↓ biotransformation
↓ esterase activity, ↓ receptor affinity
Anaesthetic management:
- Intravenous agents: ↑ dosage
- Opiates: ↑ dosing at the beginning of ECC, ↓ dosing in hypothermia and post-reventilation
- Halogens:↑ Fi due to increased solubility, but ↓ MAC in hypothermia
- Curares: ↑ effect and duration due to ↓ receptor affinity, risk of decurarisation at ECC start
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- ANTOGNINI J. Hypothermia eliminates isoflurane requirements at 20 degrees celsius. Anesthesiology 1993; 78:1152-7
- BAILEY JM, LEVY JH, KIKURA M. Pharmacokinetics of intravenous milrinone in patients undergoing cardiac surgery. Anesthesiology 1994; 81:616-22
- BARRY AE, CHANEY MA, LONDON MJ. Anaesthetic management during cardiopulmonary bypass: a systematic review. Anesth Analg 2015; 120:749-69
- BARVAIS L, HEITZ D, SCHMARTZ D, et al. Pharmacokinetic model-driven infusion of sufentanil and midazolam during cardiac surgery: Assessment of the prospective predictive accuracy and the quality of anesthesia. J Cardiothorac Vasc Anesth 2000; 14:402-8
- BARVAIS L, RAUSIN I, GLEN JB, et al. Administration of propofol by target-controlled infusion in patients undergoing coronary artery surgery. J Cardiothorac vasc Anesth 1996; 10:877-83
- BOVILL J, SEBEL P. Pharmacokinetics of high-dose fentanyl: A study in patients undergoing cardiac surgery. Br J Anaesth 1980; 52:795-9
- DASTA J, JACOBI J, WU LS. Loss of nitroglycerin to cardiopulmonary bypass apparatus. Crit Care Med 1983; 11:50-2
- FLEZZANI P, ALVIS J, JACOBS J. Sufentanil disposition during cardiopulmonary bypass. Can J Anaesth 1987; 34:566-72
- FLYNN P, HUGHES R, WALTON B. Use of atracurium in cardiac surgery involving cardiopulmonary bypass with induced hypothermia. Br J Anaesth 1984; 56:967-82
- LANDOW L, WILSON J. An improved lidocaine infusion protocol for cardiac surgical patients. J Cardiothorac Vasc Anesth 1991; 5:209-13
- MAITRE P, FUNK B, CREVOISIER C, et al. Pharmacokinetics of midazolam in patients recovering from cardiac surgery. Eur J Clin Pharmacol 1989; 37:161-6
- METZ B, REICH NT, MELLAS N, et al. Desflurane pharmacokinetics during cardiopulmonary bypass. J Cardiothorac vasc Anesth 2001; 15:179-82
- MOORE RA, GELLER EA, GALLAGHER JD, et al. Effect of hypothermic cardiopulmonary bypass on nitroprusside metabolism. Clin Pharmacol Ther 1985; 37:680-3
- RIDDINGTON DW, VENKATESH B, BOIVIN CM, et al. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA 1996; 275:1007-12
- ROSEN D, ROSEN K. Elimination of drugs and toxins during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1997; 11:337-40
- STERN R, WEISS C, STEINBACH J, et al. Isoflurane uptake and elimination are delayed by absorption of anesthetic by the Scimed membrane oxygenator. Anesth Analg 1989; 69:657-62
- WIESENACK C, WIESNER G, KEYL C, et al. In-vivo uptake and elimination of isoflurane by different membrane oxygenators during cardiopulmonary bypass. Anesthesiology 2002; 97:133-8
- WITHINGTON D, MENARD G, HARRIS J, et al. Vecuronium pharmacokinetics and pharmacodynamics during hypothermic cardiopulmonary bypass in infants and children. Can J Anaesth 2000; 47:1188-95